��Ŀ����
17����Ȼ����һ����Ҫ�������Դ�ͻ���ԭ�ϣ���1����Ȼ������Ҫ�ɷ�ΪCH4����H2S��CO2��N2�����ʣ����ð�ˮ������Һ�ɴ���H2S���ʣ��������£�

��д��NH3�ĵ���ʽ
 ��
����д������Һ�����Ļ�ѧ����ʽ2NH4HS+O2=2NH3•H2O+2S����
��2����CH4��ԭ������������������������Ⱦ��
��֪��

д��CH4��ԭNO���Ȼ�ѧ����ʽCH4��g��+4NO��g��=2N2��g��+CO2��g��+2H2O��l����H=-1250.3kJ•mol-1��
��3����Ȼ����һ����Ҫ��;����ȡH2����ԭ��Ϊ��CO2��g��+CH4��g��?2CO��g��+2H2��g��
���ܱ�������ͨ�����ʵ���Ũ�Ⱦ�Ϊ1mol/L��CH4��CO2����һ�������·�����Ӧ�����CH4��ƽ��ת����
���¶ȼ�ѹǿ�Ĺ�ϵ��ͼ1��ʾ��
��ѹǿP1С��P2������ڡ���С�ڡ��������ɸ÷�Ӧ��Сѹǿ��ƽ�������ƶ�����ͼ��֪���¶�һ��ʱ��P1��CH4��ת���ʽϴ�����P1��P2��
�ڼ���1050��ʱ�÷�Ӧ�Ļ�ѧƽ�ⳣ��81��
��4��ͼ2��CO2�����ԭΪCH4�Ĺ���ԭ��ʾ��ͼ

д��ͭ�缫����ĵ缫��ӦʽCO2+8e-+8H+=CH4+2H2O��
���� ��1����NH3���ڹ��ۻ�������������Ӽ��������д���3�Թ��õ��Ӷԣ���ԭ�������Ϊ8�����ӣ�
������Һ�����ķ�ӦΪNH4HS��������Ӧ�������ʺ�һˮ�ϰ������ԭ���غ�͵����غ���ƽ��д��ѧ����ʽ��
��2����ͼ��ֱ�д���Ȼ�ѧ����ʽ����ϸ�˹���ɼ���õ���ӦCO2��g��+CH4��g��?2CO��g��+2H2��g�����Ȼ�ѧ����ʽ��
��3��CO2��g��+CH4��g��?2CO��g��+2H2��g����Ӧ�������������ķ�Ӧ��
���¶�һ����ͼ�������֪P1��P2ѹǿ�¼���ת����P1��˵��P1��P2��
��ͼ��1050��Cʱ����ת����75%�������ʱ��ƽ��Ũ�ȣ�1050��ʱ�÷�Ӧ�Ļ�ѧƽ�ⳣ��K=$\frac{������ƽ��Ũ���ݴη��˻�}{��Ӧ��ƽ��Ũ���ݴη��˻�}$��
��4�����������������ж�ͭ�缫Ϊ������������̼�õ��������ɼ��飬���缫Ϊ����������������ʧ��������������
��� �⣺��1����NH3���ڹ��ۻ�������������Ӽ��������д���3�Թ��õ��Ӷԣ���ԭ�������Ϊ8�����ӣ������ĵ���ʽΪ ��
��
�ʴ�Ϊ�� ��
��
������Һ�����ķ�ӦΪNH4HS��������Ӧ�������ʺ�һˮ�ϰ�����Ӧ�Ļ�ѧ����ʽΪ��2NH4HS+O2=2NH3•H2O+2S����
�ʴ�Ϊ��2NH4HS+O2=2NH3•H2O+2S����
��2����CH4��g��+2O2��g��=CO2 ��g��+2H2O��l����H=-890.3KJ/mol��
��N2��g��+O2��g��=2NO��g����H=+180KJ/mol
��˹���ɼ����-�ڡ�2�õ�CH4��g��+4NO��g��=2N2��g��+CO2 ��g��+2H2O��l����H=-1250.3kJ•mol-1 ��
�ʴ�Ϊ��CH4��g��+4NO��g��=2N2��g��+CO2 ��g��+2H2O��l����H=-1250.3kJ•mol-1 ��
��3����CO2��g��+CH4��g��?2CO��g��+2H2��g����Ӧ�������������ķ�Ӧ���÷�Ӧ��Сѹǿ��ƽ�������ƶ�����ͼ��֪���¶�һ��ʱ��P1��CH4��ת���ʽϴ�P1��P2��
�ʴ�Ϊ��С�ڣ��÷�Ӧ��Сѹǿ��ƽ�������ƶ�����ͼ��֪���¶�һ��ʱ��P1��CH4��ת���ʽϴ�����P1��P2��
��ͼ��1050��Cʱ����ת����75%��������м�����ʽ���㣬
CO2��g��+CH4��g��?2CO��g��+2H2��g��
��ʼ����mol/L�� 1 1 0 0
�仯����mol/L�� 0.75 0.75 1.5 1.5
ƽ������mol/L�� 0.25 0.25 1.5 1.5
K=$\frac{1��{5}^{2}��1��{5}^{2}}{0.25��0.25}$=81��
�ʴ�Ϊ��81��
��4�����������������ж�ͭ�缫Ϊ������������̼�õ��������ɼ��飬���缫Ϊ����������������ʧ��������������ͭ�缫�Ϸ�ɽ�ĵ缫��ӦΪ��CO2+8e-+8H+=CH4+2H2O��
�ʴ�Ϊ��CO2+8e-+8H+=CH4+2H2O��
���� ���⿼�������ʽṹ���Ȼ�ѧ����ʽ��д��˹���ɵļ���Ӧ�ã�ͼ���������ѧƽ��Ӱ�����ء�ƽ�ⳣ���ļ����֪ʶ�㣬���ջ����ǽ���ؼ�����Ŀ�Ѷ��еȣ�

| A�� | ���NaCl������Һ�����Ƶý����� | |
| B�� | �ں������������п�飬�ɼ�������ĸ�ʴ���� | |
| C�� | Ѱ�Ҹ�Ч����������̫���ֽܷ�ˮ����ȡ�����Ľ����뷽�� | |
| D�� | MgO���۵�ܸߣ��������������²��� |
| A�� | ����������ɫ���ٸı� | B�� | ���������ܶȲ��ٸı� | ||
| C�� | ��������ѹǿ���ٸı� | D�� | ��������ƽ�������������ٸı� |
 �״���һ�ֿ�������Դ�����й㷺�Ŀ�����Ӧ��ǰ����
�״���һ�ֿ�������Դ�����й㷺�Ŀ�����Ӧ��ǰ������1����ҵ��һ������������ַ�Ӧ�ϳɼ״���
��Ӧ��CO��g��+2H2��g��?CH3OH��g����H 1
��Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H 2
�ٱ����������Ƿ�Ӧ���ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ����K����
| �¶� | 250�� | 300�� | 350�� |
| K | 2.041 | 0.270 | 0.012 |
��ij�¶��£���2molCO��6molH2����2L���ܱ������У���ַ�Ӧ���ﵽƽ����c��CO��=0.2mol/L����CO��ת����Ϊ80%����ʱ���¶�Ϊ250�棨���ϱ���ѡ��
��2����֪�ڳ��³�ѹ�£�
��2CH3OH��l��+3O2��g��=2CO2��g��+4H2O��g����H1=-1275.6kJ/mol
��2CO ��g��+O2��g��=2CO2��g����H2=-566.0kJ/mol
��H2O��g��=H2O��l����H3=-44.0kJ/mol
д���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ��CH3OH��l��+O2��g��=CO��g��+2 H2O��l����H=-442.8kJ•mol-1
��3��ijʵ��С�����ݼ״�ȼ�յķ�Ӧԭ���������ͼ��ʾ�ĵ��װ�ã�
�ٸõ�������ĵ缫��ӦΪO2+2H2O+4e-=4OH-
�ڸõ���ܷ�Ӧ�����ӷ���ʽΪ2CH3OH+3O2+4OH-=2CO32-+6H2O
�۹���һ��ʱ������Һ��pH��С�����������С�����䡱����
| A�� | c��B2-��+c��HB-��=0.1 mol/L | B�� | c��B2-��+c��HB-��+c��H2B��=0.1 mol/L | ||
| C�� | c��OH-��=c��H+��+c��HB-��+2c��H2B�� | D�� | c��Na+��+c��OH-��=c��H+��+c��HB-�� |

 �ֱ��ʾN2��H2��NH3������������ڹ����������ϳɰ��Ĺ��̿�����ͼ4��ʾ��
�ֱ��ʾN2��H2��NH3������������ڹ����������ϳɰ��Ĺ��̿�����ͼ4��ʾ��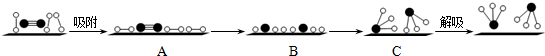
 ��һ�������£���������������������·�Ӧ��2SO2��g��+O2��g��?2SO3��g������H��0��
��һ�������£���������������������·�Ӧ��2SO2��g��+O2��g��?2SO3��g������H��0��


