��Ŀ����
16�������£���������������ʵ���Ũ�ȵ�NH4HCO3��NaCl��Һ��ϣ���������NaHCO3���壮����ָ����Һ����Ũ�ȴ�С�Ƚ���ȷ���ǣ�������| A�� | ԭNH4HCO3��Һ�У�c��HCO3-����c��NH4+����c��OH-����c��H+�� | |
| B�� | ԭNH4HCO3��Һ�У�c��OH-��=c��H+��-c��CO32-��-c��NH3•H2O��+c��H2CO3�� | |
| C�� | ������������Һ�У�c��Cl-��=c��NH4+����c��Na+��=c��HCO3-�� | |
| D�� | ������������Һ�У�c��H+��+c��H2CO3��=c��OH-��+c��CO32-��+c��NH3•H2O�� |
���� A��NH4HCO3��Һ��Һ�Լ��ԣ�HCO3-��ˮ��̶ȴ���NH4+��ˮ��̶ȣ�
B�����������غ�͵���غ������
C��NH4+��HCO3-�ᷢ��ˮ�⣻
D�����������غ�͵���غ������
��� �⣺A��NH4HCO3��Һ��Һ�Լ��ԣ�HCO3-��ˮ��̶ȴ���NH4+��ˮ��̶ȣ���c��NH4+����c��HCO3-��������c��NH4+����c��HCO3-����c��OH-����c��H+������A����
B��ԭNH4HCO3��Һ�У�����غ�Ϊ��c��NH4+��+c��Na+��+c��NH4+��=c��OH-��+2c��CO32-��+c��HCO3-��+c��Cl-����
��Һ�������غ�Ϊc��NH4+��+c��NH3•H2O��+c��Na+��=c��CO32-��+c��HCO3-��+c��H2CO3��+c��Cl-������c��OH-��=c��H+��-c��CO32-��-c��NH3•H2O��+c��H2CO3������B��ȷ��
C��NH4+��HCO3-�ᷢ��ˮ�⣬����c��Cl-����c��NH4+����c��Na+����c��HCO3-������C����
D��������������Һ�����ʿ��Կ���NH4Cl��NaHCO3����Һ�е���غ�Ϊ��c��NH4+��+c��Na+��+c��NH4+��=c��OH-��+2c��CO32-��+c��HCO3-��+c��Cl-����
��Һ�������غ�Ϊc��NH4+��+c��NH3•H2O��+c��Na+��=c��CO32-��+c��HCO3-��+c��H2CO3��+c��Cl-������c��OH-��=c��H+��-c��CO32-��-c��NH3•H2O��+c��H2CO3������D��ȷ��
��ѡBD��
���� ���⿼��������Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ��еȣ���ȷ�ε�ˮ��ԭ��Ϊ���ؼ���ע�����յ���غ㡢�����غ�ĺ��弰Ӧ�÷���������������ѧ���ķ������������Ӧ��������

 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�| A�� | H+��Ba2+��Cl-��AlO2- | B�� | Fe3+��SO42-��Na+��OH- | ||
| C�� | Na+��OH-��SiO32-��Cl- | D�� | Na+��HCO3-��SO42-��OH- |
| A�� | ������ˮ�ĵ����һ����ǿ����� | |
| B�� | ǿ�������ˮ��Һ����������ʽ���� | |
| C�� | ������ˮ�ĵ����һ����������� | |
| D�� | ǿ�������Һ�ĵ�����һ�����������ǿ |
| ��ѧ�� | C-H | C-F | H-F | F-F |
| ����/��kJ•mol-1�� | 414 | 489 | 565 | 155 |
| A�� | +485 kJ•mol-1 | B�� | -485 kJ•mol-1 | C�� | +1940 kJ•mol-1 | D�� | -1940 kJ•mol-1 |
����������ѿ��
�ڼ��ѣ�C2H6O���� �Ҵ�
�۵�������ά��
��
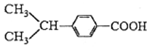 ��
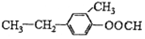
��
| A�� | �٢ڢۢ� | B�� | �٢ڢ� | C�� | �٢ڢ� | D�� | �ڢۢ� |
| A�� | �������е�������ƫ��������Һ��AlO2-+H++H2O=Al��OH��3�� | |
| B�� | ��NaClO��Һ��ͨ������SO2��2ClO-+SO2+H2O�T2HClO+SO32- | |
| C�� | Cl2ͨ��ˮ�У�Cl2+H2O�T2H++Cl-+ClO- | |
| D�� | �ù�����ˮ���չ�ҵβ���е�SO2��2NH3•H2O+SO2�T2NH4++SO32-+H2O |
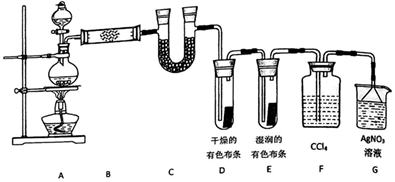
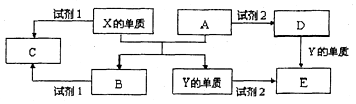 A��B��C��D��E����ѧ������5�ֻ����A��B�������Ԫ��X��Y�ĵ����������г����Ľ�����������ʼ�Ĺ�ϵ��ͼ��ʾ��
A��B��C��D��E����ѧ������5�ֻ����A��B�������Ԫ��X��Y�ĵ����������г����Ľ�����������ʼ�Ĺ�ϵ��ͼ��ʾ��