��Ŀ����
5��ij���л������л���A������ʽC3H7OCl���ϳ���֯�����̿�������������ƺϳ�·����ͼ�����ַ�Ӧ�Լ�������δע������
��֪������Ϣ��
�����������D����Է���������90��110֮�䣬����Ԫ�ص���������Ϊ61.5%��E��D����Է���������28���˴Ź���������ʾE��������2�ֲ�ͬ��������ԭ�ӣ��������Ϊ3��1��
��
 ��R��R�䣬R�������ͬ����ͬ��������
��R��R�䣬R�������ͬ����ͬ���������ش��������⣺
��1��A�����к��������ŵ��������ǻ���G��H�ķ�Ӧ��������ȥ��Ӧ��
��2��D�ķ���ʽΪC3H4O4��E�Ľṹ��ʽΪCH3OOCCH2COOCH3��
��3��H��J��Ӧ�Ļ�ѧ����ʽ��
 ��
����4��J��һ�������¿��Ժϳɸ߷��ӻ�����ø߷��ӻ�����Ľṹ��ʽ��
 ��
����5����֪1mol E��2mol J��Ӧ����1mol M�Ľṹ��ʽ��
 ��
����6��д����A������ͬ�����ŵ�A������ͬ���칹��Ľ��Լ�ʽ��
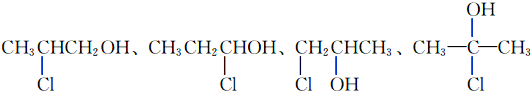 ��
��
���� D����Է���������90��110֮�䣬����Ԫ�ص���������Ϊ0.615��������ԭ�ӵĸ�������$\frac{90��0.615}{16}$��$\frac{110��0.615}{16}$֮�䣬������ԭ�ӵ���Ŀ��4��D����Է�������=$\frac{16��4}{0.615}$=104����A��D��̼ԭ�Ӹ������䣬��D�ķ���ʽΪC3H4O4��C����������ӦȻ���ữ�õ�D����D�к����Ȼ���D�IJ����Ͷ�=$\frac{3��2+2-4}{2}$=2��˵��D�к�������˫����D�ͼ״���Ӧ����E��E�к���������E��D����Է���������28���˴Ź���������ʾE��������2�ֲ�ͬ��������ԭ�ӣ��������Ϊ3��1����DΪ��Ԫ�ᣬ����D�ṹ��ʽΪHOOCCH2COOH��E�ṹ��ʽΪCH3OOCCH2COOCH3��
C����������ӦȻ���ữ�õ�D����C�ṹ��ʽΪOHCCH2CHO��B��������������C��BΪCH2OHCH2CH2OH��A�Ǻ��ȵ��л�����A�ķ���ʽ֪��A�ṹ��ʽΪCH2ClCH2CH2OH������FΪCH2ClCH2CHO��GΪCH2ClCH2COOH��G������Ӧ����H��H����������Ӧ����J��J��һ�������¿��Ժϳɸ߷��ӻ����˵��J�к���̼̼˫������G������ȥ��Ӧ����H��HΪCH2�TCHCOOH��JΪCH2�TCHCOOCH3���ݴ˷������
��� �⣺D����Է���������90��110֮�䣬����Ԫ�ص���������Ϊ0.615��������ԭ�ӵĸ�������$\frac{90��0.615}{16}$��$\frac{110��0.615}{16}$֮�䣬������ԭ�ӵ���Ŀ��4��D����Է�������=$\frac{16��4}{0.615}$=104����A��D��̼ԭ�Ӹ������䣬��D�ķ���ʽΪC3H4O4��C����������ӦȻ���ữ�õ�D����D�к����Ȼ���D�IJ����Ͷ�=$\frac{3��2+2-4}{2}$=2��˵��D�к�������˫����D�ͼ״���Ӧ����E��E�к���������E��D����Է���������28���˴Ź���������ʾE��������2�ֲ�ͬ��������ԭ�ӣ��������Ϊ3��1����DΪ��Ԫ�ᣬ����D�ṹ��ʽΪHOOCCH2COOH��E�ṹ��ʽΪCH3OOCCH2COOCH3��
C����������ӦȻ���ữ�õ�D����C�ṹ��ʽΪOHCCH2CHO��B��������������C��BΪCH2OHCH2CH2OH��A�Ǻ��ȵ��л�����A�ķ���ʽ֪��A�ṹ��ʽΪCH2ClCH2CH2OH������FΪCH2ClCH2CHO��GΪCH2ClCH2COOH��G������Ӧ����H��H����������Ӧ����J��J��һ�������¿��Ժϳɸ߷��ӻ����˵��J�к���̼̼˫������G������ȥ��Ӧ����H��HΪCH2�TCHCOOH��JΪCH2�TCHCOOCH3��
��1��A�ṹ��ʽΪCH2ClCH2CH2OH���京���������������ǻ���G������ȥ��Ӧ����H��
�ʴ�Ϊ���ǻ�����ȥ��Ӧ��
��2��ͨ�����Ϸ���֪��D�ķ���ʽΪC3H4O4��E�Ľṹ��ʽΪCH3OOCCH2COOCH3��
�ʴ�Ϊ��C3H4O4��CH3OOCCH2COOCH3��
��3��H����������Ӧ����J����Ӧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��4��JΪCH2�TCHCOOCH3����һ���������ܷ����Ӿ۷�Ӧ���ɸ߷��ӻ������ṹ��ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��5����������Ϣ֪��1mol E��2mol J��Ӧ����1mol M�Ľṹ��ʽ��  ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��6����A������ͬ�����ŵ�A������ͬ���칹��Ľ��Լ�ʽΪ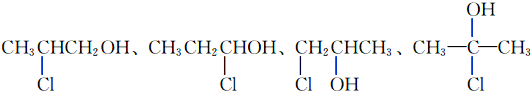 ���ʴ�Ϊ��
���ʴ�Ϊ��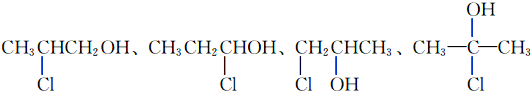 ��
��
���� ���⿼���л����ƶϣ�Ϊ��Ƶ���㣬���ؿ���ѧ�������ƶ��������漰�л����ƶϡ���Ӧ�����жϡ�ͬ���칹�����д��֪ʶ�㣬��ȷ���������ż������ʹ�ϵ��������Ӧ���ͼ���Ӧ�����ǽⱾ��ؼ������ݷ�Ӧ������������Ϣ���������ϵķ��������ƶϣ��ѵ���ͬ���칹�������жϣ���Ŀ�Ѷ��еȣ�

 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�| A�� | ���� | B�� | ˮ�� | C�� | ���� | D�� | ��ʯ�� |
| A�� | �������ڽв���ȫ���� | B�� | �������ࣺ������� | ||
| C�� | ��18����16���� | D�� | ����Ԫ��λ�ڱ������²� |
 �����£���0.10mol•L-1NaOH��Һ�ֱ�ζ�20.00mLŨ�Ⱦ�Ϊ0.10mo1•L-1��HA��Һ��HB��Һ�����õζ�������ͼ��ʾ������˵����ȷ���ǣ�������
�����£���0.10mol•L-1NaOH��Һ�ֱ�ζ�20.00mLŨ�Ⱦ�Ϊ0.10mo1•L-1��HA��Һ��HB��Һ�����õζ�������ͼ��ʾ������˵����ȷ���ǣ�������| A�� | HA������ǿ��HB | B�� | d����Һ�У�c��Na+����c��OH-����c��B-����c��H+�� | ||
| C�� | a��b����Һ�У�c��B-����c��A-�� | D�� | b����Һ�У�c��B-��+2c��OH��-=c��HB��+2c��H+�� |
| ʵ���� | HA���ʵ���Ũ�ȣ�mol/L�� | NaOH���ʵ���Ũ�ȣ�mol/L�� | �����Һ��pH |
| �� | 0.1 | 0.1 | pH=9 |
| �� | c | 0.2 | pH=7 |
| �� | 0.2 | 0.1 | pH��7 |
��1���Ӣ������������HA��ǿ�ỹ���������ᣨ�ǿ�ᡱ�����ᡱ����
��2������ʵ�����û����Һ����ˮ�������c��OH-��=10-5mol•L-1��
��3���������������c����0.2mol/L��ѡ����ڡ�����С�ڡ����ڡ��������Һ������Ũ��c��A-����c��Na+���Ĵ�С��ϵ�ǵ��ڣ�
��4���Ӣ���ʵ����������˵��HA�ĵ���̶ȴ���NaA��ˮ��̶ȣ�ѡ����ڡ�����С�ڡ����ڡ������û����Һ������Ũ���ɴ�С��˳����c��A-����c��Na+����c��H+����c��OH-����
| A�� | Ũ���� | B�� | �Ȼ�������Һ | C�� | ��ҵ���� | D�� | �廯�� |
 �絼�ʿ����ں����������Һ����������С�������£���0.100mol•L-lNH3•H2O�ζ� 10.00mLŨ�Ⱦ�Ϊ 0.100mol•L-l HCl��CH3COOH�Ļ����Һ������������ͼ��ʾ������˵����ȷ���ǣ�������
�絼�ʿ����ں����������Һ����������С�������£���0.100mol•L-lNH3•H2O�ζ� 10.00mLŨ�Ⱦ�Ϊ 0.100mol•L-l HCl��CH3COOH�Ļ����Һ������������ͼ��ʾ������˵����ȷ���ǣ�������| A�� | �ٵ���Һ��c ��H+��Ϊ0.200 mol•L-l | |
| B�� | ����Ӧ���������û���Ƚ�������Ӧ����Һ�¶ȸߵ�Ϊ�٣��ۣ��� | |
| C�� | pH�Ĵ�СΪ���ۣ��ڣ��٣��Ң۵���Һ����c��Cl-����c��CH3COO-�� | |
| D�� | �۵����������Ŀ����ʹ��Һ�絼���Խ��� |