��Ŀ����
10�������£���ijһԪ��HA��NaOH��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH�����| ʵ���� | HA���ʵ���Ũ�ȣ�mol/L�� | NaOH���ʵ���Ũ�ȣ�mol/L�� | �����Һ��pH |
| �� | 0.1 | 0.1 | pH=9 |
| �� | c | 0.2 | pH=7 |
| �� | 0.2 | 0.1 | pH��7 |
��1���Ӣ������������HA��ǿ�ỹ���������ᣨ�ǿ�ᡱ�����ᡱ����
��2������ʵ�����û����Һ����ˮ�������c��OH-��=10-5mol•L-1��
��3���������������c����0.2mol/L��ѡ����ڡ�����С�ڡ����ڡ��������Һ������Ũ��c��A-����c��Na+���Ĵ�С��ϵ�ǵ��ڣ�
��4���Ӣ���ʵ����������˵��HA�ĵ���̶ȴ���NaA��ˮ��̶ȣ�ѡ����ڡ�����С�ڡ����ڡ������û����Һ������Ũ���ɴ�С��˳����c��A-����c��Na+����c��H+����c��OH-����
���� ��1���������ʵ�����ȣ���Ϊǿ�ᣬ��Ӧ����Һ�����ԣ���Ϊ���ᣬ��Ӧ����Һ�ʼ��ԣ�
��2������Һ�������Ӻ����������Ӿ���ˮ����ģ�����pH��Kw���㣻
��3��HAΪ���ᣬ�������Ũ�Ȼ����Һ��pH����7����Ϊ��֤pH=7��Ӧʹ��Ũ�ȴ���0.2mol/L�����ݵ���غ������
��4���ɢ���ʵ������֪����Ϻ�ΪHA��NaA�Ļ��Һ��pH��7����ĵ�������ε�ˮ�⣬�������ӵ�Ũ�ȱȽϴ�С��
��� �⣺��1��һԪ��HA��NaOH��Һ�������ϣ�������Һ�����ʵ���Ũ�ȶ�Ϊ0.1mol/L�����������ʵ�����ȣ���a=7��˵����Ӧ����Һ�����ԣ���HA��ǿ�ᣬ��a��7����Ӧ��ʼ��ԣ���HA�����ᣬpH=9��˵��HA�����
�ʴ�Ϊ�����
��2�������Һ��pH=9����c��H+��=10-9mol/L������c��OH-��=10-5mol/L��NaA��Һ������������ȫ����ˮ����ģ�������ˮ�������c��OH-��=10-5mol/L��
�ʴ�Ϊ��10-5��
��3��HAΪ���ᣬ�������Ũ�Ȼ����Һ��pH����7����Ϊ��֤pH=7��Ӧʹ��Ũ��Ӧ�ô���0.2mol/L����Һ�е���غ�Ϊ��c��Na+��+c��H+��=c��A-��+c��OH-����pH=7����c��H+��=c��OH-��������c��Na+��=c��A-����
�ʴ�Ϊ�����ڣ����ڣ�
��4���ɢ���ʵ������֪����Ϻ�ΪHA��NaA�Ļ��Һ��pH��7����ĵ�������ε�ˮ�⣬���������ԣ�c��H+����c��OH-�����������ˮ�⣬��c��A-����c��Na+����c��H+����c��OH-����
�ʴ�Ϊ�����ڣ�c��A-����c��Na+����c��H+����c��OH-����
���� ���⿼������ϵ��жϣ���Ŀ�Ѷ��еȣ�����ע������������ݣ���������ʵĵ��������ˮ��ĽǶȽ��ѧϰ��ע����ع��ɺͷ����Ļ��ۣ������ڿ���ѧ���ķ��������ͶԻ���֪ʶ��Ӧ��������

| A�� | NH3 | B�� | HCl | C�� | Cl2 | D�� | NO2 |
| A�� | ���ԣ�HClO4��H2SO4��H3PO4��H2SiO3 | B�� | �ȶ��ԣ�HF��H2O��H2S | ||
| C�� | ���ԣ�KOH��NaOH��Mg��OH��2 | D�� | �۷е㣺Rb��K��Na��Li |
��Ƭ��Fe-2e-�TFe2+�� ̼����2H++2e-�TH2��
�����ж���ȷ���ǣ�������
| A�� | ��Һ����������ǿ | B�� | ̼�����ܽ� | ||
| C�� | ��Һ��H+����Ƭ�������ƶ� | D�� | ��Ƭ�Ǹ����������� |
| A�� | FeCl2 | B�� | Na2SO4 | C�� | NaOH | D�� | AlCl3 |

 ��R��R�䣬R�������ͬ����ͬ��������
��R��R�䣬R�������ͬ����ͬ�������� ��
�� ��
�� ��
��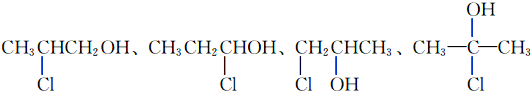 ��
��