��Ŀ����
16�� �絼�ʿ����ں����������Һ����������С�������£���0.100mol•L-lNH3•H2O�ζ� 10.00mLŨ�Ⱦ�Ϊ 0.100mol•L-l HCl��CH3COOH�Ļ����Һ������������ͼ��ʾ������˵����ȷ���ǣ�������
�絼�ʿ����ں����������Һ����������С�������£���0.100mol•L-lNH3•H2O�ζ� 10.00mLŨ�Ⱦ�Ϊ 0.100mol•L-l HCl��CH3COOH�Ļ����Һ������������ͼ��ʾ������˵����ȷ���ǣ�������| A�� | �ٵ���Һ��c ��H+��Ϊ0.200 mol•L-l | |
| B�� | ����Ӧ���������û���Ƚ�������Ӧ����Һ�¶ȸߵ�Ϊ�٣��ۣ��� | |
| C�� | pH�Ĵ�СΪ���ۣ��ڣ��٣��Ң۵���Һ����c��Cl-����c��CH3COO-�� | |
| D�� | �۵����������Ŀ����ʹ��Һ�絼���Խ��� |
���� A�����������ᣬ��ˮ�в�����ȫ���룬���Բ��ܸ������Ũ��ȷ��������Ũ�ȣ�
B������кͷ�Ӧ�Ƿ��ȷ�Ӧ�������ǡ����ȫ��Ӧʱ������ࣻ
C�����Ű�ˮ�ļ�����Һ��pH�����۵���Һ��CH3COO-ˮ��Ũ�ȼ�С��
D���۵�����Ű�ˮ�ļ��룬��Һ���������������Ũ�ȼ�С��
��� �⣺A�����������ᣬ��ˮ��ֻ�в��ֵ��룬����������Ũ��С�ڴ����Ũ�ȣ����Ըû����Һ��������Ũ��С��0.200 mol/L����A����
B������кͷ�Ӧ�Ƿ��ȷ�Ӧ�������ǡ����ȫ��Ӧʱ������࣬����ˮ�����Ϊ20mLʱǡ����ȫ��Ӧ�����۵���Һ���¶���ߣ��ٵ㻹û�з����кͷ�Ӧ����Һ�¶���ͣ����Է�Ӧ����Һ�¶ȸߵ�Ϊ�ۣ��ڣ��٣���B����
C�����Ű�ˮ�ļ��룬��ˮ���ᷴӦ���������ӣ���Һ��pH�����۵���Һ�У�����Ϊ��Ũ�ȵĴ�������Ȼ�泥�CH3COO-ˮ��Ũ�ȼ�С�������Ӳ�ˮ�⣬����c��Cl-����c��CH3COO-������C��ȷ��
D���۵�����Ű�ˮ�ļ��룬��Һ���������������Ũ�ȼ�С��������Һ�絼���Խ��ͣ���Һ�����ӵ���Ŀ�����࣬��D����
��ѡC��
���� ���⿼����������ʵĵ��롢����Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ��еȣ���ȷ��Һ�ĵ絼��������Ũ���йأ�Ũ��Խ��絼��Խ���ǽ���Ĺؼ��������ڿ���ѧ���Ի���֪ʶ��Ӧ��������

 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д� ��У���˿��ֿ���ϵ�д�
��У���˿��ֿ���ϵ�д�| A�� | �������ʵ�����HCl��Na2CO3��CH3COOH�Ļ����Һ | |
| B�� | 0.01mol•L-1�������pH=12������������Һ�������� | |
| C�� | 0.05mol•L-1��H2SO4��Һ��0.1mol•L-1��KOH��Һ�������� | |
| D�� | �ǵ��������ˮ�õ�����Һ |
��1������������裮��ʵ���з�Ӧ�������Լӿ��ԭ�������AC��
A����Ӧ���ȵ����¶����ߡ��� B��ѹǿ����
C��������Ĵ����á�������������D����Ӧ��Ӵ��������
��2������̽���ⶨʵ������в�ͬʱ����Һ���¶ȣ���������
| ʱ��/min | 0 | 5 | 10 | 15 | 20 | 25 | 35 | 50 | 60 | 70 | 80 |
| �¶�/�� | 25 | 26 | 26 | 26 | 26 | 26 | 26.5 | 27 | 27 | 27 | 27 |
��3����һ��̽�������������˽��ѧ��Ӧ�IJ�����м������ܶԷ�Ӧ�д����ã�Ϊ�ˣ������������ʵ����ƣ��������ʵ��Ŀ�IJ�����������
| ʵ�� ��� | ͭƬ ����/g | 0.1mol•L-1 ����/mL | ����ͭ ��Һ/mL | �������� ��Һ/mL | ˮ����� /mL | ʵ��Ŀ�� |
| �� | 5 | 20 | 0 | 0 | 0.5 | ʵ��ٺ͢�̽��Cu2+��ʵ���Ӱ�죻ʵ��ٺ͢�̽�����������Ӱ�죮 |
| �� | 5 | 20 | 0.5 | 0 | 0 | |
| �� | 5 | 20 | 0 | X | 0 |
| A�� | 10% | B�� | 18% | C�� | 20% | D�� | 40% |
| A�� | �����£�ͬŨ�ȵ�һԪ��HA��HB���룬����ͬŨ�ȵ�NaA��Һ��NaB��Һ��pH�� | |
| B�� | 0.2 mol/LNH4NO3 �� 0��l mol/L NaOH ��Һ�������Ϻ�c��NH4+����c ��NO3-����c ��Na+����c ��OH-����c ��H+�� | |
| C�� | ���ʵ���Ũ����ȵĴ�����Һ������������Һ�������ϣ�c ��Na+��+c��H+��=c ��CH3COO-��+c ��OH-��+c ��CH3COOH�� | |
| D�� | ͬŨ�ȵ�������Һ����NH4Al��SO4��2��NH4Cl ��NH3•H2O ��CH3COONH4������c��NH4+�� �ɴ�С��˳���ǣ��٣��ڣ��ܣ��� |

| A�� | ��Ӧ��Ļ�ѧ����ʽ��Fe3O4+CO?3FeO+CO2 | |
| B�� | ��Ӧ��Ļ�ѧ����ʽ��3FeO+H2O��g��?Fe3O4+H2 | |
| C�� | �ܷ�Ӧ�Ļ�ѧ����ʽ��C+2H2O��g��?CO2+2H2 | |
| D�� | �������������ѽ�ˮ�Ĵ��� |

 ��R��R�䣬R�������ͬ����ͬ��������
��R��R�䣬R�������ͬ����ͬ�������� ��
�� ��
�� ��
��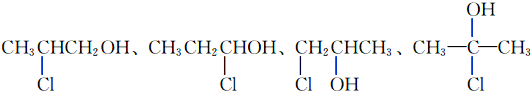 ��
�� CuSO4•5H2O��ͭ����Ҫ��������Ź㷺��Ӧ�ã�����ͭ�ڸ����·ֽ�ɵõ�SO2��SO3��O2��Cu2O��
CuSO4•5H2O��ͭ����Ҫ��������Ź㷺��Ӧ�ã�����ͭ�ڸ����·ֽ�ɵõ�SO2��SO3��O2��Cu2O��