��Ŀ����
16��ʵ�����ù���NaOH����0.5mol/L��NaOH��Һ500mL����������������Ʒ�У����ձ� ��100mL��Ͳ ��500mL����ƿ ��ҩ�� �ݲ����� ��������ƽ�������룩��1������ʱ������Ҫʹ�õ���������Ʒ�Тڣ�����ţ�����ȱ�ٵ������ǽ�ͷ�ιܣ�
��2�����в�����ʹ���Ƶ���ҺŨ��ƫ�͵���AB������ĸ��
A��û�н�ϴ��Һת�Ƶ�����ƿ B��ת�ƹ�������������Һ����
C������ƿϴ����δ���� D������ʱ���ӿ̶���
��3��������ƿʹ�÷����У����в�������ȷ���ǣ�����ţ�AC
A������ƿ������ˮϴ�������ü�Һ��ϴ
B��ʹ������ƿǰ������Ƿ�©ˮ
C�����������ƹ��������ƽ���̵���ֽ�ϣ�ȷ�����������ձ����ܽ������ע������ƿ��
D�����ݺ�����ƿ������ʳָ��סƿ��������һֻ�ֵ���ָ��סƿ�ף�������ƿ��תҡ��
��4������ʵ������������ȷ˳���ǣ�����ţ������ظ����٢ۢݢڢܣ�
����������ƽ����10g NaOH�������С�ձ��У�����������ˮ�ܽ⣻
�ڼ���������ƿ�м�����ˮ��Һ���̶���1-2cm�������ý�ͷ�ι�С�ĵμ�����ˮ
����Һ��Һ�����ʹ���̶������У�
�۰ѻָ����µ���ҺС�ĵ�ת��500mL����ƿ�У�
�ܽ�����ƿ���������ҡ�ȣ�
������������ˮϴ���ձ��벣����2-3�Σ�ϴ��Һһ��ת�Ƶ�����ƿ�У�
��5��ʵ���л���Ҫ2mol/L��NaOH��Һ850mL������ʱӦѡ�õ�����ƿ�Ĺ��ͳ�ȡNaOH�������ֱ���A������ţ�
A��1000mL��80g B��950mL��76g C��������72g D��500mL��42g��
���� ��1����������Һ�IJ�������ѡ������������
��2���������������ʵ����ʵ��������Һ�������Ӱ�죬����c=n/v�����жϣ�
��3��A������ƿ���ô�����Һ��ϴ��
B������ƿ��ʹ��ǰҪ����Ƿ�©ˮ��
C�����������и�ʴ�ԣ�
D���Ǻ�ƿ������ʳָ��סƿ��������һֻ����סƿ�ף�������ƿ��ת��ҡ�����Σ�Ŀ����ʹ��Һ��ֻ�ϣ�Ũ�Ⱦ��ȣ�
��4������������Һ�IJ���������в���˳�������
��5��ʵ����û��950mL����ƿ����Ӧѡ��1000mL����ƿ������n=cv������������Ƶ����ʵ������ٸ���m=nM���������������Ƶ�������
��� �⣺��1�����������г������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬��ȴ��ת�Ƶ�500mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ����Ի���Ҫ������Ϊ��ͷ�ιܣ��ò���100mL��Ͳ��
�ʴ�Ϊ���ڣ���ͷ�ιܣ�
��2��A��û�н�ϴ��Һת�Ƶ�����ƿ���������Ƶ�ʵ��������С����ҺŨ��ƫ�ͣ���A��ȷ��
B��ת�ƹ�������������Һ�������������Ƶ�ʵ��������С����ҺŨ��ƫ�ͣ���B��ȷ��
C�������������ˮ���ݣ�����ƿϴ����δ������Ӱ�죬��C����
D������ʱ���ӿ̶��ߣ��ᵼ����Һ���ƫС����ҺŨ��ƫ�ߣ���D����
��ѡAB��
��3��A������ƿ������ˮϴ�������ô�����Һ��ϴ���ᵼ����ҺŨ��ƫ��A����
B������ƿ��ʹ��ǰҪ����Ƿ�©ˮ�������ƺ�Ҫҡ�ȣ���B��ȷ��
C�����������и�ʴ�ԣ�Ӧ��С�ձ��г�������C����
D���Ǻ�ƿ������ʳָ��סƿ��������һֻ����סƿ�ף�������ƿ��ת��ҡ�����Σ�Ŀ����ʹ��Һ��ֻ�ϣ�Ũ�Ⱦ��ȣ���D��ȷ��
�ʴ�Ϊ��AC��
��4���ɣ�1�����������֪����ȷ˳���Ǣ٢ۢݢڢܣ�
�ʴ�Ϊ���٢ۢݢڢܣ�
��5��ʵ����û��950mL����ƿ����Ӧѡ��1000mL����ƿ�����������Ƶ�����Ϊ1L��2mol•L-1��40g/mol=80.0g��
�ʴ�Ϊ��1000��80.0g��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ��Ѷ��еȣ�����c=n/v������Һ����ԭ������������ע���������ƾ��и�ʴ�ԡ��׳��⣬Ӧ���ձ���Ѹ�ٳ�����

 �����ߴ���ϵ�д�
�����ߴ���ϵ�д�| A�� | 2��3-����-3��3-���һ����� | B�� | 2-��-3-�һ����� | ||
| C�� | 2��3-����-1-��ϩ | D�� | 2��3-����-1-��Ȳ |
| A�� | ����NaOH��Һʱ���������õ�NaOH�������С�ձ����ܽ⣬δ����ȴ����ת�Ƶ�����ƿ�в����� | |
| B�� | ת�Ƶ�����ƿ�����У���������Һ���� | |
| C�� | ת�ƺ�δϴ��С�ձ��Ͳ����� | |
| D�� | ����ʱ���ӿ̶��� |
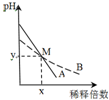 �����£�pH���1������һԪ����ҺA��B���ֱ��ˮϡ��ʱ����Һ��pH�仯��ͼ��ʾ������˵����ȷ���ǣ�������
�����£�pH���1������һԪ����ҺA��B���ֱ��ˮϡ��ʱ����Һ��pH�仯��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | ϡ��ǰ��c��A����10 c��B�� | |
| B�� | �к͵�Ũ�ȵ����������ʱ��B�õ�����϶� | |
| C�� | M��ʱA��B��Ũ����� | |
| D�� | ��M�㣬A��B���ּ���Һ�������ӵ����ʵ���Ũ����� |
| A�� | V=0ʱ��[H+]��[Cl-]=[CH3COOH] | B�� | V=10 mLʱ��[OH-]+[CH3COO-]=[H+] | ||
| C�� | V=20 mLʱ��[Na+]=[CH3COO-]+[Cl-] | D�� | ����NaOH��Һ��pH=7ʱ��V��20 mL |
| A�� | 2 mol H2��g����1 mol I2��g�� | B�� | 3 mol HI��g�� | ||
| C�� | 2 mol H2��g����2 mol I2��g�� | D�� | 1 mol I2��g����2 mol HI��g�� |
| A�� | ������ʹ�������Һ��Ϻ���Һ�����ԣ���Һ��c��Na+��=c��Cl-�� | |
| B�� | 1 mol CH4�����к��еĹ��ۼ���Ŀ����1 mol Na2O2�����к��е��������� | |
| C�� | 80��Ĵ�ˮ��pH����25��Ĵ�ˮ��pH | |
| D�� | ���������pH������ʹ�����1mol/L��NaOH��Һ��ȫ�кͣ����ĵ�NaOH��Һ������߶� |