��Ŀ����
15��ij�����A����Al2��SO4��3��Al2O3��Fe2O3����һ�������¿�ʵ����ͼ��ʾ������֮��ı仯������Al2O3��Fe2O3������ˮ��Al2��SO4��3����ˮ������ͨ����ˮ�ܹ������Ƿֿ���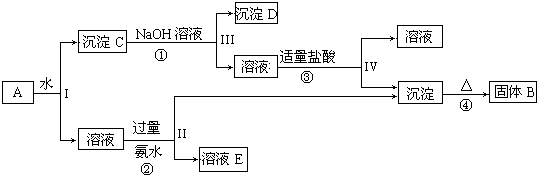
�ݴ˻ش��������⣺
��1��I��II��III��IV�IJ��ж�����Һ�ͳ����ķ����ȡ�ķ����ǹ��ˣ�
��2������������ͼ��Ӧ��ϵ��д���������ʵĻ�ѧʽ����������BAl2O3������DFe2O3����
��3��д���١��ڡ��ۡ����ĸ���Ӧ��ѧ����ʽ
��Al2O3+2NaOH+3H2O=2Na[Al��OH��4]����Al2��SO4��3+6NH3��H2O=3��NH4��2SO4+2Al��OH��3������Na[Al��OH��4]+HCl=NaCl+H2O+Al��OH��3������2Al��OH��3$\frac{\underline{\;\;��\;\;}}{\;}$Al2O3+3H2O��
��4������Ѳ�����������������Ϊ�����Ķ�����̼��Ӧ�����ӷ���ʽAlO2-+CO2+2H2O=Al��OH��3��+HCO3-��
���� Al2��SO4��3����ˮ��Al2O3��Fe2O3��������ˮ�������A��ˮ�ܽ����Һ����KAl��SO4��2������CΪAl2O3��Fe2O3��
��ת����ϵͼ��֪�������C�м�NaOH��Һ��Fe2O3����Ӧ������DΪFe2O3��Al2O3����NaOH��Һ��Ӧ����NaAlO2����NaAlO2��Һ��ͨ��CO2�ɵ�Al��OH��3������Al��OH��3���ȷֽ����ɹ���BΪAl2O3��
����Һ�мӹ�����ˮ����Һ�������ˮ��Ӧ��Al3+���������õ�����������������Һ��EΪ��NH4��2SO4�������������ᾧ���õ���NH4��2SO4��Ȼ�������ʵ����ʼ���ѧ���������
��� �⣺Al2��SO4��3����ˮ��Al2O3��Fe2O3��������ˮ�������A��ˮ�ܽ����Һ����Al2��SO4��3������CΪAl2O3��Fe2O3��
��ת����ϵͼ��֪�������C�м�NaOH��Һ��Fe2O3����Ӧ������DΪFe2O3��Al2O3����NaOH��Һ��Ӧ����NaAlO2����NaAlO2��Һ��ͨ��CO2�ɵ�Al��OH��3������Al��OH��3���ȷֽ����ɹ���BΪAl2O3��
����Һ�мӹ�����ˮ����Һ�������ˮ��Ӧ��Al3+���������õ�����������������Һ��EΪ��NH4��2SO4�������������ᾧ���õ���NH4��2SO4��
��1����Һ�ͳ����ķ������ù��ˣ��ʴ�Ϊ�����ˣ�
��2��������������֪BΪAl2O3��CΪAl2O3��Fe2O3��DΪFe2O3 �ʴ�Ϊ��Al2O3��Fe2O3��
��3����Ӧ��ΪAl2O3+2NaOH+3H2O=2Na[Al��OH��4]��
��Ӧ��ΪAl2��SO4��3+6 NH3��H2O=3��NH4��2SO4+2Al��OH��3����
��Ӧ��ΪNa[Al��OH��4]+HCl=NaCl+H2O+Al��OH��3����
��Ӧ��Ϊ2Al��OH��3$\frac{\underline{\;\;��\;\;}}{\;}$Al2O3+3H2O��
�ʴ�Ϊ��Al2O3+2NaOH+3H2O=2Na[Al��OH��4]��Al2��SO4��3+6 NH3��H2O=3��NH4��2SO4+2Al��OH��3����Na[Al��OH��4]+HCl=NaCl+H2O+Al��OH��3����2Al��OH��3$\frac{\underline{\;\;��\;\;}}{\;}$Al2O3+3H2O��
��4����NaAlO2��Һ��ͨ��CO2�ɵ�Al��OH��3��������AlO2-+CO2+2H2O=Al��OH��3��+HCO3-���ʴ�Ϊ��AlO2-+CO2+2H2O=Al��OH��3��+HCO3-��
���� ���⿼��������ƶϣ�Ϊ��Ƶ���㣬�ۺϿ���Ԫ�ػ��������ʣ�����Al��Fe���仯�������ʵĿ��飬ע�������������ԣ���ȷ�����Ļ�ѧ��ӦΪ���Ĺؼ�����Ŀ�Ѷ��еȣ�

| A�� | ��״���£�17gH2O2�к����Լ�����ĿΪNA | |
| B�� | 1L 1mo/L��Fe2��SO4��3��Һ�к��е������������Ϊ3NA | |
| C�� | ��״���£�11.2LN2��H2�Ļ������������ԭ����ΪNA | |
| D�� | 5.6gFe����1L 0.3moL/L�����У�ת�Ƶĵ�����Ϊ0.3NA |
| A�� | Al2O3��NaAlO2 | B�� | Fe��FeCl3 | C�� | Na2O��Na2CO3 | D�� | SiO2��H2SiO3 |
| A�� | ������ԭ��Ӧ�е�ʧ��������һ����� | |
| B�� | �е��ʲμӵķ�Ӧһ����������ԭ��Ӧ | |
| C�� | �������Һ���������ӵ�����һ����� | |
| D�� | �ֽⷴӦһ����������ԭ��Ӧ |
| A�� | ��״���£�14g CO����������Ϊ7NA | |
| B�� | 22gij���庬������Ϊ0.5NA������Ħ������Ϊ44 | |
| C�� | ��״���£�33.6LCH4�к�Hԭ����Ϊ6NA | |
| D�� | 1 mol Na ��ȫ��Ӧʱ��ʧȥNA������ |
 ʵ����ͨ��������ͼ��ʾ��װ������ȡ�������ش��������⣺
ʵ����ͨ��������ͼ��ʾ��װ������ȡ�������ش��������⣺