��Ŀ����
6��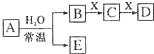 �ɶ�����Ԫ����ɵ���ѧ��������A��B��C��D��E��X ����������ͼת����ϵ������������ͷ�Ӧ������ȥ����
�ɶ�����Ԫ����ɵ���ѧ��������A��B��C��D��E��X ����������ͼת����ϵ������������ͷ�Ӧ������ȥ������1����A�ǵ��ʣ�B��D�ķ�Ӧ��OH-+HCO3-=H2O+CO32-����E��H2
��2����A�ǻ����X��Na2CO3��C�dz������������壬��ENO
��3����DΪCO��C�ܺ�E��Ӧ����A�ĵ���ʽΪ
 ����NaHCO3�ֽ���������壨ˮ�����Ͷ�����̼����������A������յõ�����ף���������500mL 1mol/L�������У�������ɫ������壬��Һ�����ԣ���A�����ʵ���Ϊ0.25mol����������ڱ�״���µ����Ϊ2.8L��������ˮ�����IJ���������������ܽ⣩��
����NaHCO3�ֽ���������壨ˮ�����Ͷ�����̼����������A������յõ�����ף���������500mL 1mol/L�������У�������ɫ������壬��Һ�����ԣ���A�����ʵ���Ϊ0.25mol����������ڱ�״���µ����Ϊ2.8L��������ˮ�����IJ���������������ܽ⣩��
���� ��1����A�ǵ��ʣ�B��D�ķ�Ӧ��OH-+HCO3-=H2O+CO32-��AΪNa��BΪNaOH��EΪH2��XΪCO2��CΪNa2CO3��DΪNaHCO3��
��2����A�ǻ����X��Na2CO3��C�dz������������壬��AΪNO2��BΪHNO3��EΪNO��CΪCO2��DΪNaHCO3��
��3����DΪCO��C�ܺ�E��Ӧ����AΪNa2O2��BΪO2��EΪNaOH��XΪC��CΪCO2��
��� �⣺��1����A�ǵ��ʣ�B��D�ķ�Ӧ��OH-+HCO3-=H2O+CO32-��AΪNa��BΪNaOH��EΪH2��XΪCO2��CΪNa2CO3��DΪNaHCO3���ʴ�Ϊ��H2��
��2����A�ǻ����X��Na2CO3��C�dz������������壬��AΪNO2��BΪHNO3��EΪNO��CΪCO2��DΪNaHCO3���ʴ�Ϊ��NO��
��3����DΪCO��C�ܺ�E��Ӧ����AΪNa2O2��BΪO2��EΪNaOH��XΪC��CΪCO2��Na2O2�ĵ���ʽΪ�� ��
��
���ȷ�����Ӧ��2NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+CO2��+H2O��������̼��������Ʒ�Ӧ����̼���ƣ�ˮ���������Ʒ�Ӧ�����������ƣ��������ᷴӦ�õ���ɫ�������壬���Ϊ�������ơ�̼���ơ��������ƣ���������500mL 1mol•L-1�������У���Һ�����ԣ���Һ������ΪNaCl�������������غ�n��Na2O2��=$\frac{1}{2}$n��NaCl�����ٸ����������غ㣬��n��Na2O2��=$\frac{1}{2}$n��NaCl��=$\frac{1}{2}$n��HCl��=$\frac{1}{2}$��0.5L��1mol/L=0.25mol��
��CԪ�ء�HԪ���غ��֪������NaOH��Na2CO3�����ʵ���֮��Ϊ2��1����������NaOH��Na2CO3�����ʵ�����Ϊ2xmol��xmol����������Ϊymol�����������غ���4x+2y=0.5����2Na2O2+4HCl=4NaCl+2H2O+O2����Na2CO3+2HCl=2NaCl+H2O+CO2������֪�����������ʵ���Ϊ����x+0.5y��mol=$\frac{1}{4}$��4x+2y��mol=$\frac{1}{4}$��0.5mol=0.125mol���ʵõ������������Ϊ0.125mol��22.4L/mol=2.8L��
�ʴ�Ϊ�� ��0.25��2.8��
��0.25��2.8��
���� ���⿼�������ƶϣ���Ҫѧ������������ѧ����������Ӧ����������Ԫ�ػ�����֪ʶ����3���м���Ϊ�״��㡢�ѵ㣬ע�������غ㷨���㣬�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | c��H+��•c��OH-��=10-14 | B�� | c��Na+��+c��K+��=c��HA-��+2c��A2-�� | ||
| C�� | c��Na+����c��K+�� | D�� | c��Na+��+c��K+��=0.05mol•L-1 |
| ������ | K+��Na+��Al3+��Cu2+ |
| ������ | OH-��HCO3-��NO3-��SO42- |
�ٽ���������ˮ��DΪ��ɫ��Һ��������Ϊ��ɫ��Һ��
�ڽ�E��Һ���뵽C��Һ�г��ְ�ɫ�����������μӣ�������ȫ�ܽ⣻
�۽�����ɫ��Ӧ��B��CΪ��ɫ������ɫ�ܲ�������A��EΪ��ɫ�����ڸ���Һ�м������ᱵ��Һ���ټӹ���ϡ���ᣬA�зų���ɫ���壬C��D�в�����ɫ������B������������
�ݽ�B��D����Һ��ϣ�δ���������������ɣ�
��������ʵ����գ�
��1��д��B��C�Ļ�ѧʽ��BKNO3�� CKAl��SO4��2��
��2��д������E���˵�C��Һ�е����ӷ�Ӧ����ʽAl3++4OH-�TAlO2-+2H2O��
��3������1mol A����Һ�뺬1mol E����Һ��Ӧ�����ɣ����õ�һ�ֻ�����û�����Ļ�ѧʽΪNa2CO3��
��4����A��Һ�м��������ij���ʯ��ˮ�������ӷ���ʽΪHCO3-+Ca2++OH-=CaCO3��+H2O��
| A�� | ú�����IJ���������쵪�� | |
| B�� | �������ڱ��Ĺ���Ԫ����Ѱ�Ұ뵼����� | |
| C�� | �������Ƶ�������ͭ���������� | |
| D�� | ���������Ʊ�Ư�� |
| ѡ�� | X | Y |
| A | SO2 | NH3 |
| B | Cl2 | CO2 |
| C | NH3 | CO2 |
| D | SO2 | Cl2 |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� |  ʵ�������Ҵ���ȡ��ϩ | B�� |  ʵ������ȡ�������� | ||
| C�� |  ���뱽�ͼױ� | D�� |  ʵ������ȡ������ |
| A�� | ���ó���ʯ��ˮ�����ֶ�����̼�Ͷ������� | |
| B�� | ����������ʳ�üӵ��Σ���KIO3���к��е�Ԫ�� | |
| C�� | ��ʹ���ȵ�CuO�ɺڱ�������һ����H2 | |
| D�� | ����ʯ����Һ�����������NaOH |

 ��
��


