��Ŀ����
2�������仯����������������ȷ�������Ҫ��Ӧ�ã��ش��������⣺��1�����к����������������ε���C�ǵ���ʵ���A��
A��NH3���� B��NH3•H2O�������� C��NH4NO3������ D��HNO3������ E��N2
��2��д��NH4NO3���뷽��ʽ��NH4NO3=NH4++NO3-����
��3��0.1mol��N2H4�����0.2molNH3���壬������ͬ�¡�ͬѹ�µ��������1��2��������ԭ��������3��4��
��4����ʾ��������+�������T���ԭ��������0.5mol${\;}_{7}^{14}$Nԭ���У��������ӵ���ĿΪ3.5NA��
��5����״���½�11.2LNH3��������ˮ�У����500mL��Һ�����ð�ˮ��Һ�����ʵ���Ũ��Ϊ1mol/L��
���� ��1����������ĸ���ѡ����ˮ��Һ�������״̬���ܵ���Ļ������ǵ���ʣ���ˮ��Һ�������״̬�¶�������Ļ������Ƿǵ���ʣ��ݴ˽�ɣ�
��2��NH4NO3Ϊ���࣬����ǿ����ʣ��ݴ���д���뷽��ʽ���ɣ�
��3�����ݰ���٤�������Լ������ۻشɣ�
��4��1mol${\;}_{7}^{14}$Nԭ���У����е����ӵ����ʵ�����7mol������N=nNA������ش�
��5������c=$\frac{n}{Vaq}$�Լ�n=$\frac{V}{Vm}$���м��㼴�ɣ�
��� �⣺��1��A��NH3�����⻯����ܵ�������ӣ����ڷǵ���ʣ�
B��NH3•H2O ���ڵ���ʣ�
C��NH4NO3�������ࣻ
D��HNO3�������������ȫ���������ӣ������
E��N2���ڵ��ʣ��Ȳ��ǵ�����ֲ��Ƿǵ���ʣ�
�ʴ�Ϊ��C��A��
��2��NH4NO3Ϊ���࣬����ǿ����ʣ����뷽��ʽΪ��NH4NO3=NH4++NO3-���ʴ�Ϊ��NH4NO3=NH4++NO3-��
��3��0.1mol��N2H4�����0.2molNH3���壬������ͬ�¡�ͬѹ�µ�����ȼ������ǵ����ʵ���֮�ȣ�0.1mol��0.2mol=1��2��������ԭ������Ϊ����1��6������2��4��=6��8=3��4���ʴ�Ϊ��1��2��3��4��
��4��1mol${\;}_{7}^{14}$Nԭ���У����е����ӵ����ʵ�����7mol������N=nNA��0.5mol${\;}_{7}^{14}$Nԭ���У��������ӵ���ĿΪ0.5��7mol��NA=3.5NA��
�ʴ�Ϊ��3.5NA��
��5��500mL���ʵ���Ũ��Ϊ1mol/L�İ�ˮ�����ʵ���Ϊ��n=c��V=1mol/L��0.5L=0.5mol��0.5mol���������V=n��Vm=0.5mol��22.4L/mol=11.2L���ʴ�Ϊ��11.2��
���� ������Ҫ����������ʵ������йؼ����Լ�������뷽��ʽ��д���ǵ���ʸ���ȣ��ѶȲ���

| A�� | I2 | B�� | MgCl2 | C�� | KCl | D�� | H2O |
| A�� | ��ɫǿ������Һ�п��ܴ������� Al3+��NH4+��Cl?��S2? | |
| B�� | ������Һ�п��ܴ������� Na+��ClO?��SO42?��I? | |
| C�� | ��������Һ�п��ܴ������� Na+��K+��Cl?��CO32? | |
| D�� | ������Һ�п��ܴ������� Ba2+��K+��Cl?��SO42? |
| A�� | �ȶ��ԣ�HCl��H2S��H2O | B�� | �ۡ��е�HF��HCl��HBr | ||
| C�� | �ۡ��е�KCl��MgO��MCl2 | D�� | �ۡ��е�CH4��SiH4��GeH4 |
| A�� |  ϡ���� | B�� |  �Ҵ� | C�� |  ϡ���� | D�� |  ϡ���� |
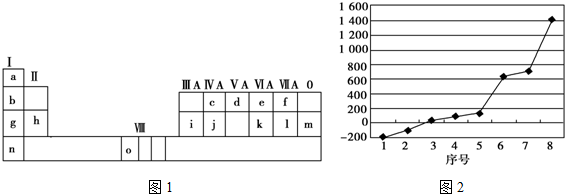
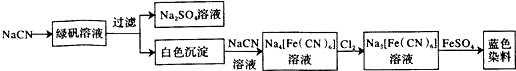
 ij��A�IJ���������������һ�����ҵ�ʯ�ͻ�����չˮƽ��A����һ��ֲ���������ڼ���A�ɷ�����ͼ��ʾ��һϵ�л�ѧ��Ӧ������ͼ�ش��������⣺
ij��A�IJ���������������һ�����ҵ�ʯ�ͻ�����չˮƽ��A����һ��ֲ���������ڼ���A�ɷ�����ͼ��ʾ��һϵ�л�ѧ��Ӧ������ͼ�ش��������⣺ ��
�� ��
��
 ��
�� ��
��
 �����������ˮ�����ӷ���ʽ2Na2O2+2H2O=4Na++4OH-+O2����0.5mol�Ĺ������CO2��Ӧת�Ƶĵ�����Ŀ0.5NA��
�����������ˮ�����ӷ���ʽ2Na2O2+2H2O=4Na++4OH-+O2����0.5mol�Ĺ������CO2��Ӧת�Ƶĵ�����Ŀ0.5NA��