��Ŀ����
 ����֪���¶ȹ���ʱ��WO2��s��ת��ΪWO2��g����
����֪���¶ȹ���ʱ��WO2��s��ת��ΪWO2��g����WO2��s��+2H2��g��?W��s��+2H2O��g����H=+66.0KJ/mol
WO2��g��+2H2��g��?W��s��+2H2O��g����H=-137.9KJ/mol
��WO2��s��?WO2��g���ġ�H=
��.1100��ʱ�������Ϊ2L�ĺ��������з������·�Ӧ��
Na2SO4��s��+4H2��g��?Na2S��s��+4H2O��g����
��1���������жϸ÷�Ӧ�ﵽƽ��״̬��������
A��������ѹǿ���� B����������ܶȲ���
C��1molH-H�����ѵ�ͬʱ�γ�2molH-O�� D��H2�������������
��2����2minʱ��Ӧ�ﵽƽ�⣬��ʱ��������������8g������H2��ʾ�÷�Ӧ�Ļ�ѧ��Ӧ����Ϊ
��3��ij�¶��¸÷�Ӧ�ﵽƽ��״̬����û�������ƽ����Է�������Ϊ14������¶��µ�ƽ�ⳣ��KΪ
��4���������¶ȣ�Kֵ��С����Ӧ�ġ�H
��5������Ӧ��ƽ�������ϵ�м���������H2����Ӧ�ٴδ�ƽ���H2O��g�����������
��ij500mL�����Һ�к�0.1molNaCl��0.1molCuSO4���ں㶨����������½��е�⣬2min��������ʼ�������壬5minʱֹͣ��⣬��ʱ�������������������������ͬ��
��д���˹����������ĵ缫��Ӧʽ
����ͼ�зֱ��������������������������ѻ��ɱ�״���µ��������ʱ��Ĺ�ϵ��
�۽���Һϡ��Ϊ1000ml��������Һ��pHΪ
���㣺�ø�˹���ɽ����йط�Ӧ�ȵļ���,��ѧƽ���Ӱ������,��ѧƽ��״̬���ж�,��ѧ��Ӧ���ʺͻ�ѧ�������Ĺ�ϵ,���ԭ��
ר�⣺�����������������
������I�����ݸ�˹���ɺ��Ȼ�ѧ����ʽ����õ������Ȼ�ѧ����ʽ��
II����1�����淴Ӧ�ﵽƽ��״̬ʱ�����淴Ӧ������ȣ�ͬ�����ʣ������淴Ӧ����֮�ȵ���ϵ��֮�ȣ���ͬ���ʣ���ƽ��ʱ�������ʵ����ʵ�����Ũ�ȵȲ��ٷ����仯���ɴ�������һЩ���������䣬�Դ˷�����
��2����������������仯����μӷ�Ӧ�����������ʵ���Ũ�ȱ仯������Ӧ���ʣ�
��3���ֱ����ƽ��ʱ������ˮ�����ʵ��������ݻ�������ƽ����Է�������������ߵĹ�ϵ���ٸ���K�ı���ʽ���㣻
��4�������¶ȶ�K��Ӱ�������
��5���÷�Ӧ�Ⱥ�ǰ������ļ�������ͬ������ϵ�м���������H2����ԭ����ƽ���ǵ�Чƽ�⣬���ݵ�Чƽ�������
III����Һ���������ӵķŵ�˳��ֱ�Ϊ�������ӷŵ�˳��Cu2+��H+��Na+�������ӷŵ�˳��ΪCl-��OH-��SO42-����������2Cl--2e-�TCl2����4OH--4e-�TO2��+H2O����������Cu2++2e-�TCu��2H++2e-�TH2�����������ӷŵ�ʵ��Ϊ���ˮ��������Һ��������NaOH�����ʵ�����ͬ�������ӹ������Դ˼��㣮
II����1�����淴Ӧ�ﵽƽ��״̬ʱ�����淴Ӧ������ȣ�ͬ�����ʣ������淴Ӧ����֮�ȵ���ϵ��֮�ȣ���ͬ���ʣ���ƽ��ʱ�������ʵ����ʵ�����Ũ�ȵȲ��ٷ����仯���ɴ�������һЩ���������䣬�Դ˷�����
��2����������������仯����μӷ�Ӧ�����������ʵ���Ũ�ȱ仯������Ӧ���ʣ�
��3���ֱ����ƽ��ʱ������ˮ�����ʵ��������ݻ�������ƽ����Է�������������ߵĹ�ϵ���ٸ���K�ı���ʽ���㣻
��4�������¶ȶ�K��Ӱ�������
��5���÷�Ӧ�Ⱥ�ǰ������ļ�������ͬ������ϵ�м���������H2����ԭ����ƽ���ǵ�Чƽ�⣬���ݵ�Чƽ�������
III����Һ���������ӵķŵ�˳��ֱ�Ϊ�������ӷŵ�˳��Cu2+��H+��Na+�������ӷŵ�˳��ΪCl-��OH-��SO42-����������2Cl--2e-�TCl2����4OH--4e-�TO2��+H2O����������Cu2++2e-�TCu��2H++2e-�TH2�����������ӷŵ�ʵ��Ϊ���ˮ��������Һ��������NaOH�����ʵ�����ͬ�������ӹ������Դ˼��㣮
���
�⣺I����WO2��s��+2H2��g��?W��s��+2H2O��g������H=+66.0kJ?mol-1
��WO2��g��+2H2��g��?W��s��+2H2O��g������H=-137.9kJ?mol-1
���ݸ�˹���ɢ�-�ڵõ�WO2��s��?WO2��g���ġ�H=+203.9kJ/mol��
�ʴ�Ϊ��+203.9 kJ?mol-1��
II����1��A����Ӧǰ����������ʵ������䣬��������ѹǿʼ�ղ��䣬���Բ��ܸ���ѹǿ�ж�ƽ��״̬����A����
B����������������䣬��Ӧ������������������Ի�������ܶ����������������ܶȲ���ʱ����ƽ��״̬����B��ȷ��
C��1molH-H������Ϊ�����ʣ��γ�2molH-O��Ϊ�����ʣ����������ʣ������ж�ƽ��״̬����C����
D�����ŷ�Ӧ���У�����������С������H2�����������С���������������������ʱ����ƽ��״̬����D��ȷ��
�ʴ�Ϊ��BD��
��2����μӷ�Ӧ�����������ʵ���Ϊxmol��
Na2SO4��s��+4H2��g��?Na2S��s��+4H2O��g��
xmol xmol
��֪2minʱ��Ӧ�ﵽƽ�⣬��ʱ��������������8g����18x-2x=8�����x=0.5��v��H2��=
=0.125mol/��L?min����
�ʴ�Ϊ��0.125mol/��L?min����
��3����ƽ��ʱ���������ʵ���Ϊamol��H2O�����ʵ���Ϊbmol����
=14����b=3a������K=
=
=81���ʴ�Ϊ��81��
��4���������¶ȣ�Kֵ��С����ƽ�����ƣ��淽��Ϊ���ȷ�������������Ϊ���ȷ�����Ӧ�ġ�H��0���ʴ�Ϊ������
��5���÷�Ӧ�Ⱥ�ǰ������ļ�������ͬ������ϵ�м���������H2����ԭ����ƽ���ǵ�Чƽ�⣬���Դﵽƽ��ʱ������İٷֺ������䣬��H2O��g��������������䣬�ʴ�Ϊ�����䣻
III������Һ���������ӵķŵ�˳��ֱ�Ϊ�������ӷŵ�˳��Cu2+��H+��Na+�������ӷŵ�˳��ΪCl-��OH-��SO42-������������2Cl--2e-�TCl2����4OH--4e-�TO2��+H2O��
�ʴ�Ϊ��2Cl--2e-�TCl2����4OH--4e-�TO2��+H2O��
��2min֮ǰ����������2Cl--2e-�TCl2����
0.1mol 0.1 0.05
��������Cu2++2e-�TCu
0.05 0.1
2min��5min��
��������4OH--4e-�TO2��+H2O
4x x
��������Cu2++2e-�TCu
0.05 0.1
2H++2e-�TH2��
4x-0.1 2x-0.05��
�������������������������ͬ����0.05+x=2x-0.05�����x=0.1�����������ռ�0.15mol���壬
��ͼ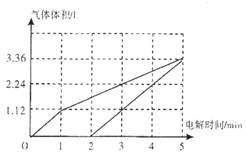 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
����Һ�з����ĵ���ܷ���Ϊ��2NaCl+2CuSO4+2H2O
H2��+O2��+Cl2��+Cu+2NaHSO4
�������0.1molNaHSO4��ϡ����1000mL����c��H+��=
=0.1mol/L������pH=1��
�ʴ�Ϊ��1��
��WO2��g��+2H2��g��?W��s��+2H2O��g������H=-137.9kJ?mol-1
���ݸ�˹���ɢ�-�ڵõ�WO2��s��?WO2��g���ġ�H=+203.9kJ/mol��
�ʴ�Ϊ��+203.9 kJ?mol-1��
II����1��A����Ӧǰ����������ʵ������䣬��������ѹǿʼ�ղ��䣬���Բ��ܸ���ѹǿ�ж�ƽ��״̬����A����
B����������������䣬��Ӧ������������������Ի�������ܶ����������������ܶȲ���ʱ����ƽ��״̬����B��ȷ��
C��1molH-H������Ϊ�����ʣ��γ�2molH-O��Ϊ�����ʣ����������ʣ������ж�ƽ��״̬����C����
D�����ŷ�Ӧ���У�����������С������H2�����������С���������������������ʱ����ƽ��״̬����D��ȷ��
�ʴ�Ϊ��BD��
��2����μӷ�Ӧ�����������ʵ���Ϊxmol��
Na2SO4��s��+4H2��g��?Na2S��s��+4H2O��g��
xmol xmol
��֪2minʱ��Ӧ�ﵽƽ�⣬��ʱ��������������8g����18x-2x=8�����x=0.5��v��H2��=
| ||
| 2min |
�ʴ�Ϊ��0.125mol/��L?min����
��3����ƽ��ʱ���������ʵ���Ϊamol��H2O�����ʵ���Ϊbmol����
| 2a+18b |
| a+b |
| c4(H2O) |
| c4(H2) |
(
| ||
(
|
��4���������¶ȣ�Kֵ��С����ƽ�����ƣ��淽��Ϊ���ȷ�������������Ϊ���ȷ�����Ӧ�ġ�H��0���ʴ�Ϊ������
��5���÷�Ӧ�Ⱥ�ǰ������ļ�������ͬ������ϵ�м���������H2����ԭ����ƽ���ǵ�Чƽ�⣬���Դﵽƽ��ʱ������İٷֺ������䣬��H2O��g��������������䣬�ʴ�Ϊ�����䣻
III������Һ���������ӵķŵ�˳��ֱ�Ϊ�������ӷŵ�˳��Cu2+��H+��Na+�������ӷŵ�˳��ΪCl-��OH-��SO42-������������2Cl--2e-�TCl2����4OH--4e-�TO2��+H2O��
�ʴ�Ϊ��2Cl--2e-�TCl2����4OH--4e-�TO2��+H2O��
��2min֮ǰ����������2Cl--2e-�TCl2����
0.1mol 0.1 0.05
��������Cu2++2e-�TCu
0.05 0.1
2min��5min��
��������4OH--4e-�TO2��+H2O
4x x
��������Cu2++2e-�TCu
0.05 0.1
2H++2e-�TH2��
4x-0.1 2x-0.05��
�������������������������ͬ����0.05+x=2x-0.05�����x=0.1�����������ռ�0.15mol���壬
��ͼ
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
������Һ�з����ĵ���ܷ���Ϊ��2NaCl+2CuSO4+2H2O
| ||
�������0.1molNaHSO4��ϡ����1000mL����c��H+��=
| 0.1mol |
| 1L |
�ʴ�Ϊ��1��
���������⿼�����Ȼ�ѧ����ʽ��˹���ɵļ���Ӧ�á���Ӧ�ٶȼ�ƽ�ⳣ���ļ��㡢Ӱ��ƽ���ƶ������ء����ԭ����Ӧ�õȣ���Ŀ�ۺ��Խ�ǿ�������֪ʶ��϶࣬�ѶȽϴ���ȷK�ĺ��塢��Чƽ��ԭ������������ӵķŵ�˳�缫��ӦΪ������Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
2008��6��1�����ҹ�ʵ�С����������Ŀ����Ϊ�˷�ֹ��������
| A����ɫ��Ⱦ | B��������Ⱦ |
| C��������Ⱦ | D����������Ⱦ |
��һ�������µ��ܱ������У����з�Ӧ��2SO2��g��+O2��g��?2SO3��g���������й�˵����ȷ���ǣ�������
| A��������ʹ�ÿ�ʵ��SO2��ת����Ϊ100% |
| B���ﵽ��ѧƽ��ʱ�������ʵ�Ũ�Ȳ��ٸı� |
| C�������������䣬�����¶ȣ���������Ӧ������ |
| D�������������䣬����SO3��Ũ�ȣ���������Ӧ������ |
���и��������У���Ϊͬ���칹����ǣ�������
| A��CH3-CH2-CH3��CH3-CH2-CH2-CH3 |
B��CH3һCH=CH-CH3�� |
| C��CH3-CH=CH-CH3��CH3-CH2-CH=CH2 |
D�� |
 Ϊ����������ʣ���������ȱ��Ӱ����������������ҹ����� ��ȡʳ�μӵ��ʩ���ݱ������˴�ʳ������ȡ������ڼ�״���л���������ͨ��һϵ�л�ѧ��Ӧ���γɼ�״���أ���״���صĽṹ��ͼ���ش��������⣺
Ϊ����������ʣ���������ȱ��Ӱ����������������ҹ����� ��ȡʳ�μӵ��ʩ���ݱ������˴�ʳ������ȡ������ڼ�״���л���������ͨ��һϵ�л�ѧ��Ӧ���γɼ�״���أ���״���صĽṹ��ͼ���ش��������⣺