��Ŀ����
14�� ��ij���������������As2O3��As2O5������ͼ����Ϣ�ش��������⣺
��ij���������������As2O3��As2O5������ͼ����Ϣ�ش��������⣺��1��As2O5�ֽ�����As2O3��O2�ķ�Ӧ�У����������E��С�����������С�����䡱����ͬ������H1���䣮
��2��As2O3�ֽ�����As��O2���Ȼ�ѧ����ʽΪAs2O3��s��=2As��s��+$\frac{3}{2}$O2��g����H=+619kJ/mol��
��3��As2O3��O2��Ӧ����As2O5���Ȼ�ѧ����ʽΪAs2O3��s��+O2��g��=As2O5��s����H=-295 kJ•mol-1��
��4����1mol As2O5�ֽ�����0.4mol As2O3��1.2mol As����÷ֽ�����У������յ�������Ϊ666.4kJ��
���� ��1����������ܽ��ͷ�Ӧ�Ļ�ܣ���Ӧ���뷴Ӧ�������������������������йأ�
��2����ͼ���֪As2O3�ֽ�����As��O2�ķ�Ӧ��Ϊ+619kJ/mol��
��3����ͼ���֪As2O3����As2O5�ķ�Ӧ��Ϊ��-914kJ/mol+619kJ/mol��=-295kJ/mol��
��4��As2O5�ֽ�����0.4mol As2O3����Ϊ295.4 kJ•mol-1��0.4mol��As2O5�ֽ�����1.2mol As����Ϊ$\frac{1}{2}$��914 kJ•mol-1��1.2mol��
��� �⣺��1����������ܽ��ͷ�Ӧ�Ļ�ܣ�����E���С����Ӧ���뷴Ӧ�������������������������йأ����������Ӧ���������������������������䣬���Է�Ӧ�Ȳ��䣻
�ʴ�Ϊ����С�����䣻
��2����ͼ���֪As2O3�ֽ�����As��O2�ķ�Ӧ��Ϊ+619kJ/mol����Ӧ���Ȼ�ѧ����ʽΪ��As2O3��s��=2As��s��+$\frac{3}{2}$O2��g����H=+619kJ/mol��
�ʴ�Ϊ��As2O3��s��=2As��s��+$\frac{3}{2}$O2��g����H=+619kJ/mol��
��4����ͼ���֪As2O3����As2O5�ķ�Ӧ��Ϊ��-914kJ/mol+619kJ/mol��=-295kJ/mol�������Ȼ�ѧ����ʽΪAs2O3��s��+O2��g��=As2O5��s����H=-295 kJ•mol-1��
�ʴ�Ϊ��As2O3��s��+O2��g��=As2O5��s����H=-295 kJ•mol-1��
��4��As2O5�ֽ�����0.4mol As2O3����Ϊ295 kJ•mol-1��0.4mol��As2O5�ֽ�����1.2mol As����Ϊ$\frac{1}{2}$��914 kJ•mol-1��1.2mol����1mol As2O5�ֽ�����0.4mol As2O3��1.2mol As����Ϊ295kJ•mol-1��0.4mol+$\frac{1}{2}$��914 kJ•mol-1��1.2mol=666.4kJ��
�ʴ�Ϊ��666.4��
���� ���⿼�����Ȼ�ѧ����ʽ����д����Ӧ�ȵļ��㡢�����Է�Ӧ�Ⱥͻ�ܵ�Ӱ�죬��Ŀ�Ѷ��еȣ�ע�����ͼ���и������ݵĺ��壬����ѧ������������֪ʶǨ��Ӧ�������Ŀ��飮

 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д� һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д� Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д����� I�����������Ʊ�ϩ��
��C3H8��g��$?_{��}^{����}$ C3H6��g��+H2��g����H1
���� II���������������Ʊ�ϩ����Ͷ��ΪC3H8��CO2��
��C3H8��g��+CO2��g�� $?_{��}^{����}$ C3H6��g��+CO��g��+H2O��g����H2=165kJ•mol-1
��CO2��g��+H2��g�� $?_{��}^{����}$ CO��g��+H2O��g����H3=41kJ•mol-1
��֪��
| ��ѧ�� | C-H | C-C | C�TC | H-H |
| ����/kJ•mol-1 | 412 | 348 | 612 | 436 |
��2��ģ�ⷽ�� I�Ʊ�ϩ��������ɱ�ķ�Ӧ���У����£�ά����ϵ��ѹǿ�㶨Ϊ0.1MPa������1mol C3H8��g��ʱ���Ϊ50L���ټ���8.5molˮ������Ϊϡ�ͼ�����Ӧt���Ӵﵽƽ�⣬��ñ���0.5mol����֪����ѹ=���ʵ�����������ѹǿ��
�ټ�����¶��·�ӦI��ƽ�ⳣ��K=0.005MPa��KP����0.001��KC����
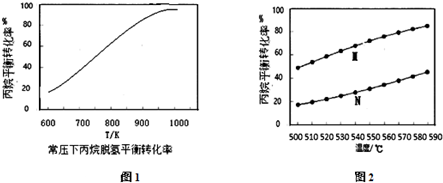
�ڳ�ѹ�£��¶�Ϊ600K��1000K��ˮ����M=10��ˮ������ָͶ����ˮ�����ͱ�������ʵ���֮�ȣ�ʱ��������ƽ��ת�������¶ȱ仯��������ͼ1����ͼ1�л���ˮ����M=8ʱ�����ߣ�
��3��ģ�ⷽ�� II�Ʊ�ϩ���ں��º��������³������ʵ���֮��Ϊ1��1�ı���Ͷ�����̼���壬һ��ʱ���ﵽƽ�⣬�����п����ж������ڷ�Ӧ��ϵ�ﵽƽ�����AB��
A��v����C3H8��=v����C3H6�� B��ƽ����Է����������ٱ仯
C�������ܶȲ��ٱ仯 D������Ͷ�����̼�����ʵ�����ֵ���ٱ仯
��4������ͬ������ģ�ⷽ�� I�뷽�� II����ñ����ƽ��ת�������¶ȵĹ�ϵ��ͼ2��ʾ��ͼ2�з��� II��Ӧ��������M���M����N�������ӻ�ѧƽ��ĽǶȽ��ͱ���ƽ��ת����M����N��ԭ�� II �ɿ����Ƿ�����Ӧ�ٺͷ�Ӧ�ۣ����ڷ�Ӧ�ۻ�����������ʹ�÷�Ӧ�ٵĻ�ѧƽ�������ƶ���
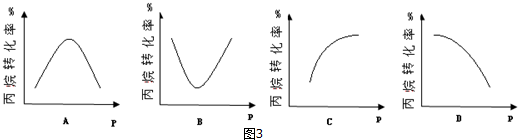
��5�����£��ܱ�������Ͷ����鷢����Ӧ�٣�ijѹǿ�·�Ӧtʱ�̺��ñ����ת���ʣ�Ȼ��������ʼʵ���������䣬�ֱ��ڲ�ͬѹǿ�£��ظ�����ʵ�飬������ͬʱ���ñ����ת������ѹǿ�仯����ͼ����ͼ3�е���ACD��
| A�� | ������ƽ | B�� | ��ͷ�ι� | C�� | 250 mL����ƿ | D�� | ��Ͳ |
| A�� | �٢� | B�� | �ڢ� | C�� | �٢� | D�� | �٢ڢ� |
 ��
�� ��
�� ��
��
