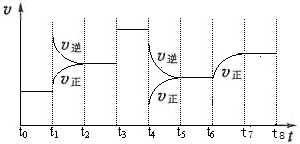
��Ŀ����
������ƿ�ϱ��У���Ũ�Ȣ��¶Ȣ�ѹǿ����������ʽ���ʽ�̶��ߣ������е�����ţ� ��
������0.1mol/L 480mL ����ͭ��Һ��Ӧ�� mL����ƿ������ȡ���ʵ��� g���� ��ȡ������CuSO4.5H2O��ʱӦע�⡰�������롱��
��������������98%���ܶ�1.84g/mL��Ũ��������100mL 1.84mol/L��ϡ���ᣬ������в��裺
��1�����㲢����Ͳ��ȡŨ������� mL��
��2���ܽ⣺�����õ�Ũ�������ձ��ڱڻ�������ʢ��ˮ���ձ��У��ӱ߽��裮
��3���� ����Һ���� mL������ƿ�У�����������ˮ��ϴ �Σ�����ϴҺ��������ƿ�У��ڲ��������в�����ʧ���Һ�壬�����ʹ��Һ��Ũ��ƫ ����ͣ���
��4��������ƿ�ڼ�ˮ���̶��� cmʱ������ С�ĵؼ�ˮ����Һ��Һ����̶������У�����ˮ�����̶��ߣ��������ҺŨ��ƫ ����ͣ�������ʱ�����ӿ̶��ߣ�Ũ�Ƚ�ƫ ����ͣ�
��5�����Ǻ�ƿ�ǣ�ҡ�Ƚ���õ���Һ�����Լ�ƿ�в����ñ�ǩ��
������0.1mol/L 480mL ����ͭ��Һ��Ӧ��
��������������98%���ܶ�1.84g/mL��Ũ��������100mL 1.84mol/L��ϡ���ᣬ������в��裺
��1�����㲢����Ͳ��ȡŨ�������
��2���ܽ⣺�����õ�Ũ�������ձ��ڱڻ�������ʢ��ˮ���ձ��У��ӱ߽��裮
��3����
��4��������ƿ�ڼ�ˮ���̶���
��5�����Ǻ�ƿ�ǣ�ҡ�Ƚ���õ���Һ�����Լ�ƿ�в����ñ�ǩ��
���㣺��Һ������
ר�⣺ʵ����
�������ٸ�������ƿ��ʹ���ص����ش�����ƿ����������һ�������һ�����ʵ���Ũ����Һ�Ķ�������������Һ�������ȷ��Ҫ��ϸߣ�ֻ���ڳ�����ʹ�ã�
�����ҳ��������50ml��100ml��150ml��200ml��500ml������ƿ������n=cv��m=nM���㵨����������
���ȸ���ϡ��ǰ�����ʵ����ʵ������䣬�����Ũ��Һ��������ٸ�������һ�����ʵ���Ũ�ȵ���Һ��Ҫ��ȡ���ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����𣮷������ɸ��� c=
�жϣ�
�����ҳ��������50ml��100ml��150ml��200ml��500ml������ƿ������n=cv��m=nM���㵨����������
���ȸ���ϡ��ǰ�����ʵ����ʵ������䣬�����Ũ��Һ��������ٸ�������һ�����ʵ���Ũ�ȵ���Һ��Ҫ��ȡ���ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����𣮷������ɸ��� c=
| n |
| v |
���
�⣺������ƿ����������һ�������һ�����ʵ���Ũ����Һ�Ķ���������ʵ���ҳ��������50ml��100ml��150ml��200ml��500ml�ȣ�����ƿ�ϱ��п̶ȡ�����������������ƿ����Һ�������ȷ��Ҫ��ϸߣ�ֻ���ڳ�����ʹ�ã�ƿ�ϱ���ʹ���¶ȣ�һ��Ϊ25�棬��ӦΪ���ڢܢޣ�
�ʴ�Ϊ�����ڢܢޣ�
�����ҳ��������50ml��100ml��150ml��200ml��500ml������ƿ����Ӧѡ��500mL����ƿ��n=0.1mol/L��0.5L=0.05mol��m=0.05mol��250g/mol=12.5g����������ƽ�������ɣ��ʴ�Ϊ��500��12.5��������ƽ��
�ۣ�1��98%��ŨH2SO4�����ʵ���Ũ��Ϊc=
=
mol/L=18.4mol/L������1.84mol/L��ϡ����100mL��
��ҪŨ��������ΪV�T
=0.01L=10mL���ʴ�Ϊ��10.0��
��3��Ũ��������ˮ���ȣ�����ȴ��ת�Ƶ�100mL����ƿ�У��ձ�������������մ�����ʣ�����ϴ��2-3�Σ�ϴ��ҺҲע������ƿ����֤����ȫ��ת�ƣ�����Һ����ʧ�������ʼ��٣�������ҺŨ��ƫ�ͣ�
�ʴ�Ϊ����ȴ��100��2-3���ͣ�
��4������ʱ����ע��ˮ���̶���1-2cmʱ��Ӧ���ý�ͷ�ιܼ�ˮ����ֹ��ˮ�����̶��ߣ�����ˮ�����̶��ߣ���v������ c=
��֪Ũ��ƫ�ͣ������ӣ�����ˮ����������̶��ߣ�v��С������ c=
��֪Ũ��ƫ�ߣ��ʴ�Ϊ��1-2����ͷ�ιܣ��ͣ��ߣ�
�ʴ�Ϊ�����ڢܢޣ�
�����ҳ��������50ml��100ml��150ml��200ml��500ml������ƿ����Ӧѡ��500mL����ƿ��n=0.1mol/L��0.5L=0.05mol��m=0.05mol��250g/mol=12.5g����������ƽ�������ɣ��ʴ�Ϊ��500��12.5��������ƽ��
�ۣ�1��98%��ŨH2SO4�����ʵ���Ũ��Ϊc=
| 1000��w |
| M |
| 1000��1.84��98% |
| 98 |
��ҪŨ��������ΪV�T
| 0.1L��1.84mol/L |
| 18.4mol/L |
��3��Ũ��������ˮ���ȣ�����ȴ��ת�Ƶ�100mL����ƿ�У��ձ�������������մ�����ʣ�����ϴ��2-3�Σ�ϴ��ҺҲע������ƿ����֤����ȫ��ת�ƣ�����Һ����ʧ�������ʼ��٣�������ҺŨ��ƫ�ͣ�
�ʴ�Ϊ����ȴ��100��2-3���ͣ�
��4������ʱ����ע��ˮ���̶���1-2cmʱ��Ӧ���ý�ͷ�ιܼ�ˮ����ֹ��ˮ�����̶��ߣ�����ˮ�����̶��ߣ���v������ c=
| n |
| v |
| n |
| v |
���������⿼��������һ�����ʵ���Ũ�ȵ���Һ��������ĿŨ���еȣ�ע����������һ�����ʵ���Ũ�ȵ���Һ��������ȷ�������ķ����뼼�ɣ�����������ѧ�����Ӧ����ѧ֪ʶ��������

��ϰ��ϵ�д�
�����Ŀ
��agij�����к��еķ�����Ϊb����cg����������ʵ���Ϊ��������
A��
| ||
B��
| ||
C��
| ||
D��
|
����ͼʵ��װ�ý�����Ӧʵ�飬�ܴﵽʵ��Ŀ���ǣ�������

| A����ͼ1��ʾװ�÷���е����ϴ�Ļ���Һ������ |
| B����ͼ2��ʾװ������FeCl2������Һ�Ʊ�FeCl2���� |
| C����ͼ3��ʾװ��ϡ��Ũ���� |
| D����ͼ4��ʾװ�÷���CCl4��ȡ��ˮ���ѷֲ���л����ˮ�� |
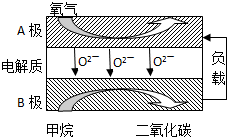 ��������Ȼ������Ҫ�ɷ֣�������������Ӧ�÷dz��㷺��һ�ֻ�ѧ���ʣ�
��������Ȼ������Ҫ�ɷ֣�������������Ӧ�÷dz��㷺��һ�ֻ�ѧ���ʣ�