��Ŀ����
19��������ϳɰ���ҵ������أ����������ǹ�ҵ��������ȡ�������Ҫ;����������м��㣺��1�����ϳɰ�ʱ������100L�ĵ����������������Ϊ1��3���Ļ������ͨ�����ϳ�����ַ�Ӧ�������Ϊ90L��������ת����Ϊ20%����д��������̣����������ðٷ�����ʾ��
��2������״���£���500L��������1Lˮ���γɰ�ˮ����˰�ˮ��������Ϊ27.5%����д��������̣����������ðٷ�����ʾ��������1λС����
��3�������������ǽ����Ϳ����Ļ����ͨ�����ȵIJ���Ͻ������ںϽ����Ĵ��£�����������һ��������NO������ʱ�¶Ⱥܸߣ�ˮ��ˮ��������ʽ���ڣ�NOҲ����O2��Ӧ�����������������ʵ���֮��Ϊ1��1.7ʱ������ת���ʿɴ�95%�����㷴Ӧ��NO���������9.8%�����������ڿ����е��������Ϊ20%��д��������̣����������ðٷ�����ʾ������1λС����
��4����һ��������������Ϊ����������������������ˮ�ɵ����ᣮΪ�ⶨij18K����Ʒ����ɣ���2.832g��Ʒ�����Ͷ��������Ũ�����У�����ܽ���ռ���NO2��N2O4�Ļ������224mL����������״������ͬ�������û��������84mL O2��Ϻ�ͨ��ˮ�У�ǡ�ñ���ȫ���գ��� ��֪������Ũ���ᣩ
��д��18K��ijɷֱ���д��������̣����������ðٷ�����ʾ������1λС�����������ý�������0����
| 18K��ɷ� | Au | Ag | Cu |
| ���������������� | 75% | 11.44% | 13.56% |
���� ��1�����ݷ�Ӧ����ʽN2+3H2?2NH3����������ı仯��Ӧ�ĵ����������Ȼ�����ת����Ϊ=$\frac{�仯��}{��ʼ��}$��
��2��n=$\frac{V}{{V}_{m}}$��m=nM����ϰ�ˮ��������Ϊ$\frac{��������}{��ˮ��������}$��
��3���ɷ�Ӧ4NH3+5O2?4NO+6H2O��g���������������Ϊ1.7�����Կ������Ϊ��8.5���������Ϊ1������ת���ʿɴ�95%�����Է�Ӧ�İ������Ϊ0.95���ɷ�Ӧ����ʽ��֪����һ�����������Ϊ0.95�����ӵ����Ϊ��$\frac{0.95}{4}$=0.238�����Է�Ӧ��NO���������Ϊ��$\frac{0.95}{8.5+1+0.238}$=9.8%��
��4������ת�Ƶ���ȷ���Ͻ��е�����Ԫ�أ��ٸ���ת�Ƶ�����ȼ��������������Ӷ�ȷ����������������������ͭ����������ȷ��п������������
��� �⣺��1�����ݷ�Ӧ����ʽN2+3H2?2NH3 ��V��
1 2
V�� 100-90
����V��=$\frac{100-90}{2}$=5L��
����ת����Ϊ=$\frac{�仯��}{��ʼ��}$=$\frac{5L}{100L��\frac{1}{4}}$=20%��
�ʴ�Ϊ��20%��
��2��n=$\frac{V}{{V}_{m}}$=$\frac{500L}{22.4L/mol}$=22.32mol������m=nM=22.32mol��17g/mol=379.46g������ˮ��������Ϊ��$\frac{379.46g}{379.46g+1000g}$=27.5%���ʴ�Ϊ��27.5%��
��3���ɷ�Ӧ4NH3+5O2?4NO+6H2O��g���������������Ϊ1.7�����Կ������Ϊ��8.5���������Ϊ1������ת���ʿɴ�95%�����Է�Ӧ�İ������Ϊ0.95���ɷ�Ӧ����ʽ��֪����һ�����������Ϊ0.95�����ӵ����Ϊ��$\frac{0.95}{4}$=0.238�����Է�Ӧ��NO���������Ϊ��$\frac{0.95}{8.5+1+0.238}$=9.8%���ʴ�Ϊ��9.8%��
��4���Ͻ�����������������=2.832g����1-75%��=0.708g��
���������ᷴӦ�Ľ���ȫ������������������=$\frac{0.0038mol��4}{1}$=1.642g��0.708g��
���������ᷴӦ�Ľ���ȫ����ͭ����ͭ������=$\frac{0.0038mol��2}{1}$=0.486g��0.708g��
��Ͻ��л���Cu��Ag��
����������Ϊm����ͭ������=0.708-m������ת�Ƶ����غ��
$\frac{0.708-m}{64g/mol}$��2+$\frac{m}{108}$=0.0038mol��4��
m=0.324g��������������=$\frac{0.324g}{2.832g}$��100%=11.44%��
��ͭ����������=25%-11.44%=13.56%��
�ʴ�Ϊ��11.44%��13.56%��
���� ���⿼�������ʵ������йؼ��㣬��������֮��Ĺ�ϵ�ǽⱾ��ؼ���ע������ڽ����е����ã�ע�⣨4���кϽ�������Ԫ�ص�ȷ���������Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�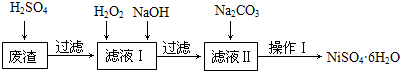
��֪��25��ʱ�����ֽ�������������ܶȻ���������ȫ������pH��Χ�����ʾ��
| Fe��OH��2 | Fe��OH��3 | Cr��OH��3 | Ni��OH��2 | |
| Ksp | 8.0��10-16 | 4.0��10-38 | 6.0��10-31 | 6.5��10-18 |
| ��ȫ����pH | ��9.6 | ��3.2 | ��5.6 | ��8.4 |
��ش��������⣺
��1�����д�ʩ���У�������߷��������ʵ���AC��
A�����߷�Ӧ�¶�B������ѹǿC���ڷ�Ӧ�����в��Ͻ���
��2������Һ���м���6%��H2O2����������2Fe2++H2O2+2H+=2Fe3++2H2O�������ӷ���ʽ��ʾ��������NaOH����pH�ķ�Χ��5.6��pH��8.4��Ϊ�˳�ȥ��Һ�е�Fe3+��Cr3+���ӣ�
��3����Һ�����Ҫ�ɷ���Na2SO4��NiSO4��
��4������Ni2+����ȫ������ʵ�鷽���Ǿ��ú����ϲ���Һ���ټ���Na2CO3��Һ��û�г������ɣ�
��5���������ʵ�鲽������Ϊ��
�ٹ��ˣ���������ˮϴ�ӳ���2��3��ֱ������Һ��pH��ֽ��������ԣ�
��������м�ϡ��H2SO4��Һ��ֱ��ǡ����ȫ�ܽ⣻
������Ũ������ȴ�ᾧ�����˵�NiSO4•6H2O���壻
���������Ҵ�ϴ��NiSO4•6H2O���岢���ɣ�
| A�� | PCl3��BCl3����������ԭ�ӵ�����㶼�ﵽ8�����ȶ��ṹ | |
| B�� | Ϊ��ǿ��ˮ��Ư���ԣ����������м���̼��� | |
| C�� | NaH����ˮ��Ӧ�ķ���ʽ��NaH+D2O�TNaOH+D2�� | |
| D�� | ������NA�����ӵ�H2O��CH4������ͬ����� |
CH4+2O2��CO2+2H2O��C3H8+5O2��3CO2+4H2O
����һ������Ȼ��Ϊȼ�ϵ���ߣ������Һ��ʯ������Ӧ��ȡ����ȷ��ʩ�ǣ�������
| A�� | ����������������Сʯ���������� | |
| B�� | �������������������ʯ���������� | |
| C�� | ��С��������������Сʯ���������� | |
| D�� | ��С����������������ʯ���������� |
| A�� | �����û����ҺΪ���ԣ���a��b=1��1 | |
| B�� | �����û����ҺΪ���ԣ������ɳ��������ʵ���Ϊ0.05b mol | |
| C�� | ��a��b=9��2����������ҺpH����2 | |
| D�� | ��a��b=9��2����û����Һ������ܽ�����0.28��a+b��g |
 �ۼ�������Ҫ�ɷ���KMnO4��H2C2O4��������ϴ����ù�ߵ����ijС��ͬѧ��̽��H2C2O4Ũ�ȶԷ�Ӧ���ʵ�Ӱ�죮
�ۼ�������Ҫ�ɷ���KMnO4��H2C2O4��������ϴ����ù�ߵ����ijС��ͬѧ��̽��H2C2O4Ũ�ȶԷ�Ӧ���ʵ�Ӱ�죮��ʵ��ԭ����2KMnO4+5H2C2O4+3H2SO4=K2SO4+2MnSO4+10CO2��+8H2O��
��ʵ����ƣ�
��1��������һ����������������ͬ�¶��£�������ͼװ�ã��ⶨ��ͬŨ��H2C2O4��Һ��KMnO4��Ӧ������ͬ���CO2�����ʱ�䣨����CO2�ܽ��Ե�Ӱ�죩����ͼ������Ӧѡ��٢ڢܢݢޣ����ţ�������װ����װ�ã���ѡ�����������ٹ��ƿ����˫��������ˮ�ۡ����ܡ��ݵ��ܡ�����Ͳ��
��2�������������۲췴Ӧ������������ͬ�����£��ⶨ��ͬŨ��H2C2O4��Һ��������Ũ��KMnO4��Һ��Ϻ���Һ��ɫ�����ʱ�䣮�ⶨʵ�����ݣ����Т١��ڴ������ݷֱ���5.0��25��
| ʵ����� | �¶�/�� | �����ʵ����/mL | ��Ӧʱ�� | |||
| H2O | 3mol/Lϡ���� | 0.1mol/L KMnO4��Һ | 0.6mol/LH2C2O4��Һ | |||
| 1 | 25 | 3.0 | 2.0 | 4.0 | 6.0 | t1 |
| 2 | 25 | �� | 2.0 | 4.0 | 4.0 | t2 |
| 3 | �� | 7.0 | 2.0 | 4.0 | 2.0 | t3 |
��3��ʵ���ҳ���KMnO4����Һ�ζ�ijŨ�Ȳ��ᣮKMnO4��Һ������ʽ�ζ��ܣ����ʽ����ʽ�����У��жϵ���ζ��յ�������ǵ��������һ��KMnO4��Һʱ����Һ����ɫ��Ϊdz�Ϻ�ɫ���Ұ�����ڲ���ɫ������ȡ�ζ�������ʱ����ʼ������ȷ���յ㸩�Ӷ�������ⶨ���ƫ�ͣ��ƫ�ߡ�����ƫ�͡�������Ӱ�족����
| A�� | CO�ǻ�ԭ�� | B�� | Fe2O3����ԭ | C�� | ����������CO2 | D�� | �����û���Ӧ |
 ��֪��A������ʯ�͵���Ҫ�л�����ԭ�ϣ������ʿ�����������һ������ʯ�ͻ�����չˮƽ��E�Ǿ��й���ζ������F��һ�ָ߾�����Ƴɶ��ְ�װ���ϣ�
��֪��A������ʯ�͵���Ҫ�л�����ԭ�ϣ������ʿ�����������һ������ʯ�ͻ�����չˮƽ��E�Ǿ��й���ζ������F��һ�ָ߾�����Ƴɶ��ְ�װ���ϣ�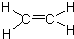 ��C������Ϊ��ȩ��
��C������Ϊ��ȩ��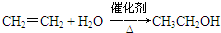 ��
�� ��
��