��Ŀ����
4����1�������½�NaOH��Һ��һԪ����HA��Һ��Ϻ��ش��������⣺
������PH=3��HA��Һ��PH=11��NaOH��Һ�������Ϻ���Һ��PHֵ��7���������=��
������Ϻ���ҺPHֵ����7�����Ϻ���Һ�и�����Ũ�ȴ�С����Ϊc��A-��=c��Na+����c��OH-��=c��H+����
��2����֪������Ksp[Al��OH��3]=3��10-34��Ksp[Fe��OH��3]=4��10-38��25����Ũ�Ⱦ�Ϊ0.1mol/L��AlCl3��FeCl3�����Һ����μ��백ˮ��
?��д���������ɳ��������ӷ���ʽFe3++3NH3•H2O=Fe��OH��3��+3NH4+��?����ҺPH=10ʱ��C��Al3+��=3��10-22mol/L
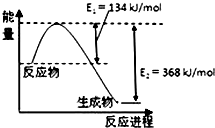
��3��1909�껯ѧ�ҹ�����ʵ�����״κϳ��˰�������Ӧ�ý϶࣬�ݴ˻ش��������⣺�ϳɰ���Ӧ�Ļ�ѧ����ʽΪN2��g��+3H2��g��?2NH3��g����
��д���÷�Ӧ��ƽ�ⳣ������ʽ��K=$\frac{{c}^{2}��N{H}_{3}��}{c��{N}_{2}����{c}^{3}��{H}_{2}��}$��
?����һ�������µ�2L�ܱ������н���ʵ�飬�������������
| ���� | N2 | H2 | NH3 |
| ��ʼ��mol�� | 2 | 7 | 0 |
| 10s��mol�� | 1.6 | ||
| ƽ�⣨mol�� | 2 |
?����һ���¶ȣ�һ��������ܱ������н��кϳɰ���ʵ�飬���в������жϷ�Ӧ�ﵽƽ��״̬��ΪAD��
A���ܶȱ��ֲ���
B��ƽ�����������ֲ���
C������������ʵ������ֲ���
D����λʱ����ÿ����1molN2��ͬʱ����2mol����
������β���к��н϶��NO2��NO�������ŷſ��γɹ⻯ѧ����������NH3�ɽ����ȥ��ͬʱ�õ�������Ⱦ���������ʣ���д��NH3��NO2��Ӧ�Ļ�ѧ��ѧ����ʽ8NH3+6NO2=7N2+12H2O��
���� ��1����pH=3��HA��ҺŨ��ԶԶ����10-3mol/L��pH=11��NaOH��ҺŨ��Ϊ10-3mol/L�����ߵ������ϵõ�HA��NaA�����Һ����HA��Ũ��Զ����NaA��Ũ�ȣ���Һ�����ԣ�
�ڻ�Ϻ���ҺpH=7������Һ��c��OH-��=c��H+������ϵ���غ��жϣ�
��2������������������������ɾ�Ϊ1��3�ͣ������������ܶȻ���С����������������������
����ҺpH=10ʱ������Ksp[Al��OH��3]=c��Al3+����c3��OH-��=3��10-34����c��Al3+����
��3���ٻ�ѧƽ�ⳣ����ָ��һ���¶��£����淴Ӧ����ƽ��ʱ���������Ũ��ϵ������֮���뷴Ӧ���Ũ��ϵ������֮���ıȣ����塢��Һ�岻��Ҫ�ڻ�ѧƽ�ⳣ����д����
�ڸ���ƽ��ʱ���������ʵ�������μӷ�Ӧ�������ʵ������������㵪��ת���ʣ�
�ۿ��淴Ӧ����ƽ��ʱ��ͬ�����ʵ�������������ұ��ֲ��䣬����ֵ�Ũ�ȡ��������ֲ��䣬�ɴ�����������һЩ�����䣬�ж�ƽ���������Ӧ�淴Ӧ���з����仯�����������ɱ仯�����ٱ仯˵������ƽ�⣻
��NH3��NO2��Ӧ�õ�������Ⱦ���������ʣ���Ӧ���ɵ�����ˮ��
��� �⣺��1����pH=3��HA��ҺŨ��ԶԶ����10-3mol/L��pH=11��NaOH��ҺŨ��Ϊ10-3mol/L�����ߵ������ϵõ�HA��NaA�����Һ����HA��Ũ��Զ����NaA��Ũ�ȣ���Һ�����ԣ�������Һ��pHֵ��7��
�ʴ�Ϊ������
�ڻ�Ϻ���ҺpH=7������Һ��c��OH-��=c��H+�����ɵ���غ㣺c��A-��+c��OH-��=c��Na+��+c��H+�����ɵã�c��A-��=c��Na+��������Һ��c��A-��=c��Na+����c��OH-��=c��H+����
�ʴ�Ϊ��c��A-��=c��Na+����c��OH-��=c��H+����
��2������������������������ɾ�Ϊ1��3�ͣ������������ܶȻ���С������������������������Ӧ���ӷ���ʽΪ��Fe3++3NH3•H2O=Fe��OH��3��+3NH4+��
����ҺpH=10ʱ��c��OH-��=10-4mol/L������Ksp[Al��OH��3]=c��Al3+����c3��OH-��=3��10-34���ɵ�c��Al3+��=3��10-22mol/L��
�ʴ�Ϊ��Fe3++3NH3•H2O=Fe��OH��3��+3NH4+��3��10-22��
��3����N2��g��+3H2��g��?2NH3��g���Ļ�ѧƽ�ⳣ������ʽK=$\frac{{c}^{2}��N{H}_{3}��}{c��{N}_{2}����{c}^{3}��{H}_{2}��}$��
�ʴ�Ϊ��$\frac{{c}^{2}��N{H}_{3}��}{c��{N}_{2}����{c}^{3}��{H}_{2}��}$��
��ƽ��ʱ���ɰ���2mol����N2��g��+3H2��g��?2NH3��g����֪�μӷ�Ӧ�ĵ���Ϊ2mol��$\frac{1}{2}$=1mol���ʵ�����ת����Ϊ$\frac{1mol}{2mol}$��100%=50%��
�ʴ�Ϊ��50%��
��A�������ݻ����䣬����������������䣬�ܶ�ʼ�ձ��ֲ��䣬��A����
B������������������䣬�淴Ӧ���л�����������ʵ�����С��ƽ����Է�����������ƽ�����������ֲ���ʱ��˵����Ӧ�ﵽƽ��״̬����B��ȷ��
C���淴Ӧ���л�����������ʵ�����С��������������ʵ������ֲ���ʱ��˵����Ӧ����ƽ��״̬����C��ȷ��
D����λʱ����ÿ����1molN2��ͬʱ����2mol����������ʾ����Ӧ��������Ӧʼ�հ��ñ�����ϵ���У���D����
��ѡ��AD��
��NH3��NO2��Ӧ�õ�������Ⱦ���������ʣ���Ӧ���ɵ�����ˮ����Ӧ����ʽΪ��8NH3+6NO2=7N2+12H2O��
�ʴ�Ϊ��8NH3+6NO2=7N2+12H2O��
���� ���⿼������Ũ�ȴ�С�Ƚϡ���Һ������жϡ��ܶȻ��йؼ��㡢��ѧƽ����㡢��ѧƽ��״̬���жϵȣ�ע��Ի���֪ʶ���������գ�ע���ж�ƽ���������Ӧ�淴Ӧ���з����仯�����������ɱ仯�����ٱ仯˵������ƽ�⣮

| A�� | HCl | B�� | Cl2 | C�� | Na2O2 | D�� | NaOH |
| ѡ�� | �������ʵ | ���� |
| A | ���ȵĴ�����Һϴȥ���� | Na2CO3��ֱ�������۷�Ӧ |
| B | �����£���������Ũ��ϡ�����зֱ������ͬ����Ƭ��Ũ��������Ƭ���ܽ��� | ��Ӧ��Ũ��Խ��Ӧ����Խ�� |
| C | ��pH�ơ��絼���ǣ�һ�ֲ�����Һ�������������������ɼ������������ˮ��̶� | ���������Ƿǵ���ʣ������ܵ��磬����ˮ����������ǵ���ʣ����ᣩ |
| D | FeCl3��Һ������ͭ��ӡˢ��·������ | FeCl3�ܴӺ�Cu2+����Һ���û���ͭ |
��0.2mol/LHCl��Һ����ˮ�������c��H+����0.2mol/LMOH��Һ����ˮ�������c��H+���� �������������������=����
����������Һ��������ʽ�ľ�ȷ����������������֣���c��Cl-��-c��M+��=9.9��10-7mol/L��
��2������������0.2mol/LMOH��Һ��0.1mol/LHCl��Һ�������ϣ���û����Һ��pH��7����˵������ͬ������MOH�ĵ���̶ȣ�MCl��ˮ��̶ȣ��������������������=����
��3������������pH=3��HR��Һ��pH=11��NaOH��Һ�������ϣ�������Һ��pH��ȷ���������7��������7��������ȷ������
��4����0.1032mol/LHCl��Һ�ζ�δ֪Ũ�ȵ�NaOH��Һ���ظ����ε�ʵ������������ʾ��
| ʵ����� | 0.1032mol/LHCl��Һ���/mL | ����NaOH��Һ���/mL |
| 1 | 27.83 | 25.00 |
| 2 | 25.53 | 25.00 |
| 3 | 27.85 | 25.00 |
�����������������ⶨ���ƫ�ߵ���BC��
A����ƿδ�ô���Һ��ϴ
B����ʽ�ζ���δ�ñ�������Һ��ϴ
C���ζ�ǰ�ζ��ܼ�������һ���ݣ��ζ���������ʧ��
D���ζ�ǰ���ζ����е���ҺҺ����͵��ڡ�0��������
��5���ζ��ķ���������к͵ζ��������ζ�����ϵζ��ȣ������ζ����õ�ָʾ����������һ�ֳ���������֪һЩ���ε���ɫ��Ksp��20�棩���£��ⶨˮ�����Ȼ���ĺ��������ñ���������Һ���еζ���
| ��ѧʽ | AgCl | AgBr | AgI | Ag2S | Ag2CrO4 |
| ��ɫ | ��ɫ | dz��ɫ | ��ɫ | ��ɫ | ��ɫ |
| Ksp | 1.8��10-10 | 5.0��10-13 | 8.3��10-17 | 2.0��10-48 | 1.8��10-10 |
A��KI B��K2CrO4 C��KBr D��K2S��

| A�� | ����Ļ�ѧʽΪHNO3 | |
| B�� | ����C��ʱ��Һ�е������������Ҫ��Ba2+��NH4+ | |
| C�� | �ձ��иռ�����ʱ���۲첻���а�ɫ�������� | |
| D�� | ��A��E����ǿ��I��С����Ҫԭ�����������ܵ��κ��ѵ����ˮ |

��֪����һ�������¿ɷ�����Ӧ��Si��s��+3HCl��g��?SiHCl3��g��+H2��g����H��0
I����Ӧ�ڡ��۾���Ҫ���ȣ������������¶�����ֱ�����Ӧѡ������Ϊ��Ӧ��Ӧѡ��a���������ĸ����ԭ������ӦSi��s��+3HCl��g��?SiHCl3��g��+H2��g����H��0���¶Ƚϵ�ʱ��Ӧ��������г̶Ƚϴ�������SiHCl3�����ɣ�
a.520��530K B��1350��1360K
II������ʵ����ģ�ҵ�ϴֹ��ᴿ�Ĺ��̣���֪SiHCl3��ˮǿ��ˮ�⣬��������������±���ʾ��
| ���� | SiCl4 | SiHCl3 | AlCl3 | FeCl3 |
| �е�/�� | 57.7 | 33.0 | - | 315 |
| �����¶�/�� | - | - | 180 | 300 |

A����HCl�ķ���װ�ã�����ΪӦѡ����������װ�ã�d����װ�õ������ĸ����װ��D�м�ʯ�ҵ�����Ϊ����ʣ��HCl����ֹ�����е�ˮ��������Cװ�����SiHCl3��ˮ�⣻

��2����֪Һ̬��ƷSiHCl3�к�������SiCl4��AlCl3��FeCl3�ȣ��������в�����Ϊ������������ƣ������в��Ǹò��������������bd����װ�������ĸ����
a�������� b�� Բ����ƿ c�� ������ƿ d�� ��Һ©�� e���¶ȼ� f��������
��3����SiHCl3��H2��Ӧ�Ʊ������װ�����£�

�ٰ�ͼʾ��װ������������ʵ�鲽�����ȷ˳��Ϊdbacef�����������ĸ����
A����װ�÷�Һ©���������μ�ϡ���ᣬ��Ӧ����H2��
B����װ��������ҩƷ��
C����װ�÷�Һ©�����������μ�SiHCl3����������Ӧװ�ã�
D�����װ�������ԣ�
e��ֹͣ���װ�õμ�SiHCl3����ֹͣ������Ӧװ�ã�
f��ֹͣͨH2��
����c����Ҫ���ȵ�װ��Ϊ����������װ����š��ס������ҡ�������������������
�ڸ���װ�õ����ȱ������β������װ�ã�
| A�� | pHС��7����ˮ����Ϊ���� | |
| B�� | �ƹ�ʹ��ȼú����������Ҫ��Ϊ�˷���SO2��Ⱦ | |
| C�� | Ϊ��ֹ��֬ʳƷ�����������ʣ����ڰ�װ���з�����ʯ�һ�轺 | |
| D�� | ��������ˮ�ɲ������������ԵĽ������ӣ�����������ˮ��ɱ������ |
 ���ǵ����Ϻ����ḻ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���ã�
���ǵ����Ϻ����ḻ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���ã�
 ��
�� ��
�� ��
�� ��E��CH3CH3��
��E��CH3CH3�� ��
��