��Ŀ����
16��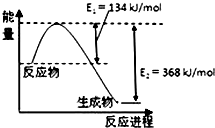 ���ǵ����Ϻ����ḻ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���ã�
���ǵ����Ϻ����ḻ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���ã���1��25��ʱ��0.1mol/LNH4NO3��Һ��ˮ�ĵ���̶ȴ��ڣ�����ڡ��������ڡ���С�ڡ��� 0.1mol/L NaOH��Һ��ˮ�ĵ���̶ȣ�
��2������0.1mol/L NaOH��Һ��0.2mol/LNH4NO3��Һ�������ϣ������Һ��2c��NH4+����c��NO3-����������Һ������Ũ���ɴ�С��˳����c��NO3-����c��NH4+����c��Na+����c��OH-����c��H+����
��3��������ʱ�£�N2H4��Ϊȼ�ϣ����������������������߷�Ӧ���ɵ�������̬ˮ�����ⶨ16g������������Ӧ�зų�284kJ��������
��÷�Ӧ���Ȼ�ѧ����ʽ��2N2H4��g��+2NO2��g��=3N2��g��+2H2O��g����H=-1136kJ/mol��
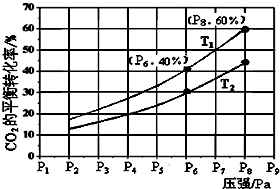
��4��ͼ��1mol NO2��1mol CO��Ӧ����CO2��NO�����������仯ʾ��ͼ��
��֪��N2��g��+O2��g��=2NO��g����H=+180kJ/mol
2NO ��g��+O2��g��=2NO2��g����H=-112.3kJ/mol
��Ӧ��2NO��g��+2CO��g��?N2��g��+2CO2��g���ġ�H=-760.3kJ/mol��
���� ��1�������������Һ�������ˮ��ٽ���ˮ�ĵ��룬����������Һ������������������ˮ�ĵ����жϣ�
��2������2c��NH4+����c��NO3-���ж��������������Ũ�ȹ�ϵ��Ȼ�������Һ��ʾ�����ж���Һ�и�����Ũ�ȴ�С��ϵ��
��3������n=$\frac{m}{M}$����16gN2H4�����ʵ������ٸ����Ȼ�ѧ����ʽ��дԭ����д�Ȼ�ѧ����ʽ��
��4�����������仯ͼ����Ӧ�ȵ�������Ӧ�Ļ�ܼ�ȥ�淴Ӧ�Ļ�ܣ��ݸ�˹���ɼ���֪��������ѧ����ʽ�Ϳ��������Ӧ���ʱ䣮
��� �⣺��1���������Һ�У�Ӧ������ӽ��ˮ��������������ӣ��ٽ���ˮ�ĵ��룬������������Һ������������������ˮ�ĵ��룬�����������Һ��ˮ�ĵ���̶ȴ�������������Һ��ˮ�ĵ���̶ȣ�
�ʴ�Ϊ�����ڣ�
��2�������Һ��2c��NH4+����c��NO3-����c��NH4+����$\frac{1}{2}$c��NO3-��=c��Na+����������Һ������Ũ�ȹ�ϵΪ��c��NO3-����c��NH4+����c��Na+����c��OH-����c��H+����
�ʴ�Ϊ��c��NO3-����c��NH4+����c��Na+����c��OH-����c��H+����
��3��16gN2H4�����ʵ���Ϊ��$\frac{16g}{32g/mol}$=0.5mol�������������Ӧ���ɵ�������̬ˮ�ų�284kJ����������1mol��������ȫȼ��������̬ˮ�ų�������Ϊ568kJ�����Ը��Ȼ�ѧ����ʽ�ǣ�2N2H4��g��+2NO2��g���T3N2��g��+4H2O��g����H=-1136kJ•mol-1��
�ʴ�Ϊ��2N2H4��g��+2NO2��g��=3N2��g��+2H2O ��g����H=-1136kJ/mol��
��4���÷�Ӧ���ʱ��H=E1-E2=134KJ/mol-368KJ/mol=-234KJ/mol�������Ȼ�ѧ����ʽΪ��NO2��g��+CO��g��=CO2��g��+NO��g����H=-234kJ•mol-1��
N2��g��+O2��g��=2NO��g����H1=+180kJ•mol-1 ��
2NO��g��+O2��g��=2NO2��g����H=-112.3kJ•mol-1 ��
NO2��g��+CO��g��=CO2��g��+NO��g����H=-234kJ•mol-1 ��
���ݸ�˹���ɣ���+�ۡ�2-�٣��û�ѧ����ʽΪ��2NO��g��+2CO��g��?N2��g��+2CO2��g����H=��-112.3kJ•mol-1 ��+��-234kJ•mol-1����2-��+180kJ•mol-1��=-760.3kJ/mol��
�ʴ�Ϊ��-760.3kJ/mol��
���� ���⿼����ˮ�ĵ��롢�Ȼ�ѧ����ʽ����д������Ũ�ȴ�С�Ƚϵ�֪ʶ����Ŀ�Ѷ��еȣ��漰��֪ʶ��϶࣬ע���˹�������Ȼ�ѧ����ʽ�е�Ӧ�ã�����������ѧ�����Ӧ����ѧ֪ʶ��������

| A�� | BeCl2 | B�� | CO2 | C�� | HCl | D�� | N2 |
��1����ˮ����ѧ������
��������ʵ��֤����ˮ���������BD������ĸ��ţ���
A����ˮ�ܸ��Ȼ�������Һ��Ӧ��������������
B�������£�0.1mol•L-1��ˮpHΪ11
C����������ֽ�
D�������£�0.1mol•L-1�Ȼ����Һ��pHΪ5
�����з����У�����ʹ��ˮ����̶��������BC������ĸ��ţ���
A��ͨ�백�� B�����������Ȼ�������
C����ˮϡ�� D�����������Ȼ�粒���
��2������ʹ�������ѧ������
��0.1mol•L-1NaOH��Һ�ֱ�ζ������Ϊ20.00mL��Ũ�Ⱦ�Ϊ0.1mol•L-1������ʹ�����Һ���õ��ζ���������ҺpH�����NaOH��Һ������仯�������ζ����ߣ�
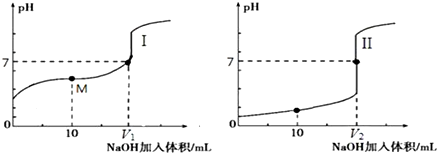
�ٵζ������������I���I����II������
�ڵζ���ʼǰ��������Һ����ˮ�������c��H+��������0.1mol•L-1������Һ��
��V1��V2�Ĺ�ϵ��V1��V2�����������=����������
��M���Ӧ����Һ�У������ӵ����ʵ���Ũ���ɴ�С��˳����c��CH3COO-����c ��Na+����c ��H+����c ��OH-����
��3��Ϊ���о������εij����ܽ�ƽ��ͳ���ת����ijͬѧ�������ʵ�飮
| ����1����2mL 0.005mol•L-1 AgNO3��Һ�м���2mL 0.005mol•L-1KSCN��Һ�����ã� | ���ְ�ɫ������ |
| ����2��ȡ1mL�ϲ���Һ���Թ��У��μ�1��2mol•L-1Fe��NO3��3��Һ�� | ��Һ��Ϊ��ɫ�� |
| ����3������2����Һ�У���������5�� 3mol•L-1AgNO3��Һ�� | ����a���ְ�ɫ��������Һ��ɫ��dz�� |
| ����4������1���µ���Һ�м���5�� 3mol•L-1KI��Һ�� | ���ֻ�ɫ������ |
����ͬ�¶��£�Ksp��AgI��=8.3��10?17��Ksp ��AgSCN ��=1.0��10?12��
�ٲ���3������a�dz��ְ�ɫ������
���ó����ܽ�ƽ��ԭ�����Ͳ���4��ʵ������AgSCN��s��?Ag+��aq��+SCN-��aq��������KI����Ϊ�ܽ�ȣ�AgSCN��AgI��Ag+��I-��Ӧ����AgI��ɫ������AgSCN���ܽ�ƽ�������ƶ���
����50mL 0.005mol•L?1��AgNO3��Һ�м���150mL0.005mol•L?1�� KSCN��Һ����Ϻ���Һ��Ag+��Ũ��ԼΪ4��10?10mol•L?1����������Һ����仯��
�ش��������⣺
������PH=3��HA��Һ��PH=11��NaOH��Һ�������Ϻ���Һ��PHֵ��7���������=��
������Ϻ���ҺPHֵ����7�����Ϻ���Һ�и�����Ũ�ȴ�С����Ϊc��A-��=c��Na+����c��OH-��=c��H+����
��2����֪������Ksp[Al��OH��3]=3��10-34��Ksp[Fe��OH��3]=4��10-38��25����Ũ�Ⱦ�Ϊ0.1mol/L��AlCl3��FeCl3�����Һ����μ��백ˮ��
?��д���������ɳ��������ӷ���ʽFe3++3NH3•H2O=Fe��OH��3��+3NH4+��?����ҺPH=10ʱ��C��Al3+��=3��10-22mol/L
��3��1909�껯ѧ�ҹ�����ʵ�����״κϳ��˰�������Ӧ�ý϶࣬�ݴ˻ش��������⣺�ϳɰ���Ӧ�Ļ�ѧ����ʽΪN2��g��+3H2��g��?2NH3��g����
��д���÷�Ӧ��ƽ�ⳣ������ʽ��K=$\frac{{c}^{2}��N{H}_{3}��}{c��{N}_{2}����{c}^{3}��{H}_{2}��}$��
?����һ�������µ�2L�ܱ������н���ʵ�飬�������������
| ���� | N2 | H2 | NH3 |
| ��ʼ��mol�� | 2 | 7 | 0 |
| 10s��mol�� | 1.6 | ||
| ƽ�⣨mol�� | 2 |
?����һ���¶ȣ�һ��������ܱ������н��кϳɰ���ʵ�飬���в������жϷ�Ӧ�ﵽƽ��״̬��ΪAD��
A���ܶȱ��ֲ���
B��ƽ�����������ֲ���
C������������ʵ������ֲ���
D����λʱ����ÿ����1molN2��ͬʱ����2mol����
������β���к��н϶��NO2��NO�������ŷſ��γɹ⻯ѧ����������NH3�ɽ����ȥ��ͬʱ�õ�������Ⱦ���������ʣ���д��NH3��NO2��Ӧ�Ļ�ѧ��ѧ����ʽ8NH3+6NO2=7N2+12H2O��
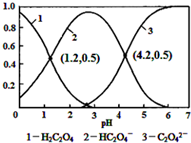
| A�� | pH=1.2��Һ�У�c��K+��+c��H+���Tc��OH-��+c��H2C2O4�� | |
| B�� | pH=2.7��Һ�У�$\frac{{c}^{2}��H{C}_{2}{O}_{4}^{-}��}{c��{H}_{2}{C}_{2}{O}_{4}��}$��c��C2O42-��=1000 | |
| C�� | ����ͬ���ʵ���KHC2O4��K2C2O4������ȫ����ˮ���û��Һ��pHΪ4.2 | |
| D�� | ��pH=1.2����Һ�м�KOH��Һ��pH������4.2�Ĺ�����ˮ�ĵ����һ������ |
 ijУ��չ�����о���ѧϰ���ӷϾɸɵ���л���̼����MnO2��NH4Cl��ZnCl2�����ʣ������������£��ش��й����⣺
ijУ��չ�����о���ѧϰ���ӷϾɸɵ���л���̼����MnO2��NH4Cl��ZnCl2�����ʣ������������£��ش��й����⣺