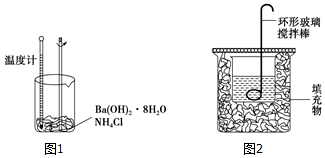
��Ŀ����
16��ijͬѧ����ʵ�������������ƹ�������0.84mol/L��NaOH��Һ95mL���ش��������⣮��1������������������������ƽ���ձ������������Լ�ƿ�⣬����Ҫ �����������ƣ�100mL����ƿ����ͷ�ιܣ�
��2����������ƽ��ȡNaOH������Ϊ3.4g��
��3������ʱ����ȷ�IJ���˳����B��C��A��F��E��D������ĸ��ʾ��ÿ����ĸֻ����һ�Σ���
A��������ˮϴ���ձ���������2��3�Σ�ϴ��Һ��ע������ƿ����
B����������ƽ���������NaOH���壬�����ձ��У��ټ�������ˮ���ò���������������ʹ����ȫ�ܽ�
C��������ȴ��NaOH��Һ�ز�����ע������ƿ��
D��������ƿ�ǽ�����ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ��Һ�����͵�ǡ����̶�������
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶���1��2cm��
��4������ͼʾ��Ӧ�IJ����淶����B
��5�������������������������Һ��Ũ�Ƚ��к�Ӱ�죨�ƫ�ߡ�����ƫ�͡�����Ӱ�족����
��û�н���A����ƫ�ͣ�
�ڳ���ʱ�����ƫ�ͣ�
��������ʱ���ӿ̶���ƫ�ߣ�
�ܶ���ҡ��ʱ������Һ���½����ּ�ˮ���̶���ƫ�ͣ�
������ƿ������ˮϴ����û�и�����Ӱ�죮
���� ��1������0.84mol/L��NaOH��Һ95mL��ѡ��100mL����ƿ�����ձ����ܽ⡢��ȴ��ת�Ƶ�����ƿ�ж��ݣ�
��2�����m=cVM���㣻
��3��������Һʱ����Ϊ���㡢�������ܽ⡢��ȴ��ת�ơ����ݡ�ҡ�ȡ�װƿ��
��4��A������ʱ�������룬�ҹ��岻�ܷ��������ϣ�
B����������������ܽ⣻
C��ת��Һ����Ҫ������������
D������ʱ��ͷ�ι��������ţ�
��5����c=$\frac{n}{V}$��֪����ϲ���������n��V��Ӱ���ж�ʹ������ҺŨ�ȱ仯���Դ������
��� �⣺��1������0.84mol/L��NaOH��Һ95mL��ѡ��100mL����ƿ�����ձ����ܽ⡢��ȴ��ת�Ƶ�����ƿ�ж��ݣ�����Ҫ������ƽ���ձ������������Լ�ƿ����ͷ�ιܵȣ��ʴ�Ϊ��100mL����ƿ����ͷ�ιܣ�
��2��������ƽ��ȡNaOH������Ϊ0.1L��0.84mol/L��40g/mol=3.36g��3.4g���ʴ�Ϊ��3.4��
��3��������Һʱ����Ϊ���㡢�������ܽ⡢��ȴ��ת�ơ����ݡ�ҡ�ȡ�װƿ����˳��ΪB��C��A��F��E��D���ʴ�Ϊ��B��C��A��F��E��D��
��4��A������ʱ�������룬��NaOH���������е�С�ձ��г�������A����
B����������������ܽ⣬������������B��ȷ��
C��ת��Һ����Ҫ������������ͼ��ȱ�ٲ���������C����
D������ʱ��ͷ�ι��������ţ�ͼ��λ�ò���������D����
�ʴ�Ϊ��B��
��5����û�н���A������n��С����c=$\frac{n}{V}$��֪��Ũ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
�ڳ���ʱ�������NaOH���⣬NaOH������ƫС��n��С����c=$\frac{n}{V}$��֪��Ũ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
��������ʱ���ӿ̶��ߣ�VƫС����c=$\frac{n}{V}$��֪��Ũ��ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
�ܶ���ҡ��ʱ������Һ���½����ּ�ˮ���̶��ߣ�Vƫ����c=$\frac{n}{V}$��֪��Ũ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
������ƿ������ˮϴ����û�и��n��V�����䣬��Ũ����Ӱ�죬�ʴ�Ϊ����Ӱ�죮
���� ���⿼������һ��Ũ�ȵ���Һ��Ϊ��Ƶ���㣬����ʵ�������������Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬ע����Ũ�ȹ�ʽ��������Ŀ�ѶȲ���

| ������ ��� ѡ�� | ������ | ����� | ����� | �ǵ���� |
| A | �մ� | �������� | CaCO3 | �ɱ� |
| B | ��� | Ư�� | NH3•H20 | Fe |
| C | ���� | ���Ͻ� | ʯī | ���� |
| D | ���� | CuSO4•5H2O | Na2SO4 | �ƾ� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | C3H6��C5H10 | B�� | �ڶ��ױ���Զ��ױ� | ||
| C�� | C2H6��C5H12 | D�� | ��������1��2-�������� |
 ��ijѧ����0.2000mol•L-1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ������ɷ�Ϊ���¼�����
��ijѧ����0.2000mol•L-1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ������ɷ�Ϊ���¼�����