��Ŀ����
13������������Ϊ36.5%��Ũ���ᣨ�ܶ�Ϊ1.16g/cm3�����Ƴ�1mol/L��ϡ����220ml���Իش��������⣺��1��Ũ��������ʵ���Ũ��Ϊ11.6mol/L��
��2�������㣬����ȡŨ����ʱ��ѡ��������Ͳ�е�C������ţ���
A��5mL B��10mL C��25mL D��50mL
��3������ȡŨ������������в�����
�ٵ�ϡ�͵��������¶�������һ�º��ز�����ע������ƿ�У�
��������ƿ��С�ļ�����ˮ��Һ��ӽ��̶���2��3cm�������ý�ͷ�ιܼ�����ˮ��ʹ��Һ�İ���ײ���ƿ���Ŀ̶������У�
����ʢ������ձ���ע������ˮ�����ò�����������ʹ���Ͼ��ȣ�
��������ˮϴ���ձ��Ͳ�����2��3�Σ�����ϴ��Һȫ��ע������ƿ��
���������У���ȷ��˳����D
A���٢ڢۢ�B�� �ۢܢ٢�
C���ܢ٢ڢ�D���ۢ٢ܢ�
��4�����������ƹ����У��øո�ϴ�ӽྻ����Ͳ����ȡŨ���ᣬ�����Ƶ�ϡ����Ũ����ƫ�ͣ��ƫ�ߡ�����ƫ�͡�������Ӱ�족����
���� ��1������C=$\frac{1000�Ѧ�}{M}$����Ũ��������ʵ���Ũ�ȣ�
��2����Һϡ�������������ʵ����ʵ������ֲ��䣬�ݴ˼�����ҪŨ�������������Ũ�������ѡ����Ͳ���
��3����������һ�����ʵ���Ũ����Һ��һ�㲽������
��4���øո�ϴ�ӽྻ����Ͳ����ȡŨ���ᵼ��Ũ���ᱻϡ�ͣ���ȡ���������Ȼ�������ʵ���ƫС������C=$\frac{n}{V}$�ж���
��� �⣺��1����������Ϊ36.5%��Ũ���ᣨ�ܶ�Ϊ1.16g/cm3�������ʵ���Ũ��C=$\frac{1000��1.16��36.5%}{36.5}$=11.6mol/L��
�ʴ�Ϊ��11.6��
��2�����Ƴ�1mol/L��ϡ����220ml��Ӧѡ��250mL����ƿ��ʵ������250mL��Һ������ҪŨ�������ΪV����Һϡ�������������ʵ����ʵ������ֲ����֪��V��11.6mol/L=1.0mol/L��250mL�����V=21.6mL��
Ӧѡ��25mL��Ͳ��
�ʴ�Ϊ��C��
��3������һ�����ʵ���Ũ����Һ��һ�㲽�裺���㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ�ȣ�������ȷ��˳��Ϊ���ۢ٢ܢڣ�
�ʴ�Ϊ��D��
��4���øո�ϴ�ӽྻ����Ͳ����ȡŨ���ᵼ��Ũ���ᱻϡ�ͣ���ȡ���������Ȼ�������ʵ���ƫС������C=$\frac{n}{V}$��֪��ҺŨ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���ȷ����ԭ���Ͳ����ǽ���ؼ���ע������ƿ����Ͳ���ѡ������ݣ���Ŀ�ѶȲ���

| A�� | CO32- | B�� | SO42- | C�� | Cl- | D�� | Na+ |
 ij��ɫ��Һ���ܺ���NH4+��Na+��Mg2+��Al3+��Fe3+��S042-��S032-��S2032-��C032һ�е��������ӣ�Ϊ��̽������ɣ��ֱ�ȡ��Һ100mL��������ʵ�飺
ij��ɫ��Һ���ܺ���NH4+��Na+��Mg2+��Al3+��Fe3+��S042-��S032-��S2032-��C032һ�е��������ӣ�Ϊ��̽������ɣ��ֱ�ȡ��Һ100mL��������ʵ�飺��������������ǣ�������
| A�� | ��Һ��һ������NH4+��C032- | |
| B�� | ��Һ������Ũ��c��Na+��=c��S042һ�� | |
| C�� | ��Һ��һ������Mg2+��Al3+��Fe3+ | |
| D�� | ��Һ��������Na2C03�루NH4��2S04��ɵĻ����Һ |
| A�� | ����ʱ�����̸ߣ����̵� | B�� | ����ʱ�����Ӷ�ȡ�̶� | ||
| C�� | ����ƿϴ����δ���� | D�� | ����ʱ��Һ�泬���˿̶��� |
| A�� | SO3��NxOy���������������� | |
| B�� | ʵ������ȡ����ʱ���ȼ��ȶ������̣������Ũ���� | |
| C�� | ����þ���ơ���ͬ�壬����̼��ƺ�̼�ᱵ�����ܣ�����̼����Ҳ���� | |
| D�� | �ṹ��������Ƶ����ʣ��е�����Է���������������ߣ�����NH3�е����PH3 |
| A�� | Na2CO3��Һ��ͨ������SO2��SO2+H2O+2CO32-�TSO32-+2HCO3- | |
| B�� | Ư���еμ�Ũ���������ȡ������ClO-+Cl-+2H+ $\frac{\underline{\;\;��\;\;}}{\;}$ Cl2+H2O | |
| C�� | ��0.1mol•L-1��NH4Al��SO4��2��Һ��0.2mol•L-1��Ba��OH��2��Һ�������ϣ�Al3++2SO42-+2Ba2++4OH-�TAlO2-��+2BaSO4��+2H2O | |
| D�� | ��0.4molFeI2����Һ��ͨ��0.3mol������4Fe2++2I-+3Cl2�T4Fe3++I2+6Cl- |
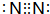 ��
��