��Ŀ����
8����������Դ�ǽ��������Ⱦ����Ҫ�ٴ룬���м״������������ʵ����ȼ�ϣ�������ȼ�ϵ�أ���1����֪����2CH3OH��l��+3O2��g���T2CO2��g��+4H2O��g����H1=-1274.0kJ/mol
��2CO��g��+O2��g���T2CO2��g����H2=-566.0kJ/mol
��H2O��g���TH2O��l����H3=-44kJ/mol
�״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽΪCH3OH��l��+O2��g��=CO��g��+2H2O��l����H=-442kJ•mol-1��
��2�������״���ԭ��CO��H2���ɷ�ӦCH4��g��+H2O��g��$\frac{\underline{\;����\;}}{\;}$CO��g��+3H2��g����H��0�õ���
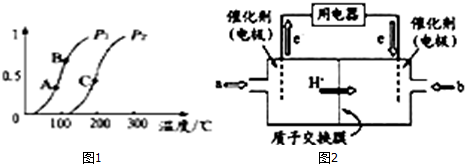
��һ��������CH4��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ1����P1��P2�����������������=������ͬ����A��B��C���㴦��Ӧƽ�ⳣ����KA��KB��KC���Ĵ�С˳��ΪKA��KB��KC��
��100��ʱ����1molCH4��2molH2Oͨ���ݻ�Ϊ1L�Ķ����ܱ������з�����Ӧ����˵���÷�Ӧ�Ѿ��ﵽƽ��״̬����ac ������ţ���
a��������ѹǿ�㶨 b�������������ܶȺ㶨
c��3v����CH4��=v����H2�� d����λʱ��������0.1molCH4ͬʱ����0.3molH2
��3���״�ȼ�ϵ�أ����DMFC�����ڽṹ������ת���ʸߡ��Ի�������Ⱦ������Ϊ������Դ�����Ʒ��Խ��Խ�ܵ���ע��DMFC����ԭ����ͼ2��ʾ��ͨ��a����ĵ缫��ԭ��صĸ��������������������b�缫��ӦʽΪO2+4e-+4H+=2H2O��
���� ��1�������Ȼ�ѧ����ʽ���ø�˹���ɼ��㷴Ӧ�Ȳ���д�Ȼ�ѧ����ʽ��
��2������200��ʱ������ͬѹǿ��CO��ת���ʴ�С����ϻ�ѧ����ʽ�з�Ӧǰ�����������仯�������Ӧ���ȣ����¶����ߣ�ƽ�������ƶ�����ѧƽ�ⳣ��K����
�ڿ��淴Ӧ�ﵽƽ��״̬ʱ�����淴Ӧ������ȸ����Ũ�ȱ��ֲ��䣻
��3������ͼ֪������Ĥ�����ӽ���Ĥ����������Һ�����ԣ������������ƶ�����֪��ͨ��a�ĵ缫Ϊ������ͨ��b�ĵ缫Ϊ�����������ϼ״�ʧȥ���ӷ���������Ӧ���������������õ����ӷ�����ԭ��Ӧ��������Һ������ˮ��
��� �⣺��1�����ݸ�˹���ɣ�����֪��Ӧ[��-��-�ۡ�4]��2�õ�CH3OH��l��+O2��g��=CO��g��+2H2O��l�������Ը÷�Ӧ�ġ�H=[��-1274.0kJ/mol��-��-566.0kJ/mol��-��-44.0kJ/mol����4]��2=442kJ•mol-1����CH3OH��l��+O2��g��=CO��g��+2H2O��l����H=-442kJ•mol-1��
�ʴ�Ϊ��CH3OH��l��+O2��g��=CO��g��+2H2O��l����H=-442kJ•mol-1��
��2������ͼa��200��λ�ã�ƽ�������ửһ�����ߣ��ɼ�CH4��ת����P1��P2����CH4��g��+H2O��g��?CO��g��+3H2��g����Ӧ�У������������������ڷ�Ӧ�ѹǿ����ʱƽ���������ƶ�����P1��P2������Ӧ���ȣ����¶����ߣ�ƽ�������ƶ�����ѧƽ�ⳣ��K��������KA��KB��KC��
�ʴ�Ϊ������KA��KB��KC��
�ڿ��淴Ӧ�ﵽƽ��״̬ʱ�����淴Ӧ������ȸ����Ũ�ȱ��ֲ��䣬
a����Ӧǰ�����������ͬ������������ѹǿ�㶨ʱ��Ӧ�ﵽƽ��״̬����a��ȷ��
b������������䣬�����������䣬���������������ܶ�ʼ�պ㶨����b����
c��3v����CH4��=v�棨H2����˵�����淴Ӧ������ȣ���Ӧ�ﵽƽ��״̬����c��ȷ��
d����λʱ��������0.1molCH4ͬʱ����0.3molH2����������Ӧ���ʣ�����˵����Ӧ�ﵽƽ��״̬����d����
�ʴ�Ϊ��ac��
��3������ͼ֪������Ĥ�����ӽ���Ĥ����������Һ�����ԣ������������ƶ�����֪��ͨ��a�ĵ缫Ϊ������ͨ��b�ĵ缫Ϊ�����������ϼ״�ʧȥ���ӷ���������Ӧ��������ӦʽΪ CH3OH-6e-+H2O=CO2+6H+�������������õ��ӷ�����ԭ��Ӧ���缫��ӦʽΪO2+4e-+4H+=2H2O��
�ʴ�Ϊ������O2+4e-+4H+=2H2O��
���� ���⺭���Ȼ�ѧ���绯ѧ�������ݣ���Ҫ��Ӱ�컯ѧƽ�����ء���ѧƽ�ⳣ���ĺ����Ӱ�����ط������漰�缫�ж���缫��Ӧʽ��д�����⣬����ʱע���������ԭ�ĽǶ��ж�ԭ��ص��������Լ��缫����ʽ����д������ԭ���ǹؼ�����Ŀ�Ѷ��еȣ�

 �����ߴ���ϵ�д�
�����ߴ���ϵ�д� H2S�����ǻ�������õ�������Ӧԭ��Ϊ��2H2S��g��+02��g���TS2��s��+2H20��1����H=-632kJ•mol-1����ͼΪ����ĤH2Sȼ�ϵ��ʾ��ͼ������˵����ȷ���ǣ�������
H2S�����ǻ�������õ�������Ӧԭ��Ϊ��2H2S��g��+02��g���TS2��s��+2H20��1����H=-632kJ•mol-1����ͼΪ����ĤH2Sȼ�ϵ��ʾ��ͼ������˵����ȷ���ǣ�������| A�� | �õ�ؿ�ʵ�ְѻ�ѧ��ȫ��ת��Ϊ���� | |
| B�� | �缫b�Ϸ����ĵ缫��ӦΪ��02+2H2O+4e-�T40H- | |
| C�� | �缫a�Ϸ����ĵ缫��ӦΪ��2H2S-4e-�TS2+4H+ | |
| D�� | ����34gH2S���뷴Ӧʱ����2mo1H+������Ĥ���˸����� |

| A�� | ͼ1��ʾ���������Ӱ�췴Ӧ���ʱ� | |
| B�� | ͼ2��ʾ��0.10mol/L CH3COOH��Һ�ζ�20.00 mL0.10mol/LNaOH��Һ���õ��ĵζ����� | |
| C�� | ͼ3��ʾKNO3���ܽ�����ߣ�ͼ�е���ʾ����Һ��80��ʱKNO3�IJ�������Һ | |
| D�� | ͼ4��ʾtʱ����Ӧ���ʴ����淴Ӧ���� |
| A�� | ����Һ�м�KSCN����Һ�Ժ�ɫ��֤��ԭ��Һ����Fe3+����Fe2+ | |
| B�� | ����ͨ����ˮCuSO4����ĩ������֤��ԭ�����к���ˮ���� | |
| C�� | ���հ�ɫ��ĩ������ʻ�ɫ��֤��ԭ��ĩ����Na+����K+ | |
| D�� | �����ó���ʯ��ˮ������Na2CO3��Һ��NaHCO3��Һ |
 ��֪2NO2�TN2O4+Q ��Q��0������һ������NO2����ע�����к��ڣ���ͼ���������ѹ��ע�����Ĺ���������������ʱ��ı仯��������ɫԽ�����ԽС��������˵����ȷ���ǣ�������
��֪2NO2�TN2O4+Q ��Q��0������һ������NO2����ע�����к��ڣ���ͼ���������ѹ��ע�����Ĺ���������������ʱ��ı仯��������ɫԽ�����ԽС��������˵����ȷ���ǣ�������| A�� | c��IJ���������ע���� | |
| B�� | b����a����ȣ�c��NO2������c��N2O4����С | |
| C�� | ����Ӧ��һ���������н��У���a��b�����ƽ�ⳣ��Ka��Kb | |
| D�� | d�㣺v��������v���棩 |
| A�� | �ŵ�ʱ���������Һ��pH���Ͻ���������������PbSO4 ���� | |
| B�� | �ŵ�ʱ��ÿͨ��1mol���ӣ����ؾ�Ҫ����2mol H2SO4 | |
| C�� | ���ʱ��������Ӧ��PbSO4+2e-=Pb+SO42- | |
| D�� | ���ʱ��Ǧ���صĸ�������ӵ�Դ�ĸ������� |
 ��ͼ��ʾ������K1�̶�����������K2�������ƶ���T��ʱ��M��N���������о�������ӦN2��g��+3H2��g��?2NH3��g����������M��N�и�����l mol N2��3mol H2����ʼM��N���ݻ����¶���ͬ���������¶Ȳ��䣮����˵���в���ȷ���ǣ�������
��ͼ��ʾ������K1�̶�����������K2�������ƶ���T��ʱ��M��N���������о�������ӦN2��g��+3H2��g��?2NH3��g����������M��N�и�����l mol N2��3mol H2����ʼM��N���ݻ����¶���ͬ���������¶Ȳ��䣮����˵���в���ȷ���ǣ�������| A�� | ��Ӧ�ﵽƽ��ʱN2��ת���ʣ�M��N | B�� | H2�����������M��N | ||
| C�� | NH3��Ũ�ȣ�M��N | D�� | �÷�Ӧ��T��ʱ��ƽ�ⳣ��K��M=N |
| A�� | ��Һ����������֮��������㣺c��Na+����c��OH-����c��H+����c��CH3COO-�� | |
| B�� | ����Һ�в������Ӽ����㣺c��CH3COO-��=c��Na+���������Һһ�������� | |
| C�� | ����Һ�����ʽ�ΪCH3COONa�������Ӽ�һ�����㣺c��Na+����c��CH3COO-����c��OH-����c��H+�� | |
| D�� | ����Һ�е�����ΪCH3COONa��CH3COOH������Һ�����Ӽ�������㣺c��CH3COO-����c��Na+����c��H+����c��OH-�� |
