��Ŀ����
п�̷ϵ�ؿɻ���п����Ԫ�������������ϼ�����п��̼���̣��������̷�ΪԤ����������п�����ߡ�̼�������������֣�����������ҵ��̼���̹������£�

��֪��c��Zn2+��=1mol/L����Һ�м���NaOH��Һ��Zn��OH��2�����������ܽ��pH���£�

�Իش��������⣺
��1����ʽп�̵�ع���ʱ������ӦʽΪ�� ��
��2������пˮ��Һ�����ԣ������ӷ���ʽ˵�� ��
��3��д��Zn��OH��2�ĵ��뷽��ʽ ��
��4������Zn2+������ȫʱ��Һ��ʣ��Zn2+Ũ��Ϊ1��10-5mol/L�����������Ksp[Zn��OH��2]= ��д����������
��5�����������е�Mn2+���ӿ����������ƣ�NaBiO3��������ˮ�����Լ������ԭ���������Խ����У�Mn2+��������MnO4-��NaBiO3����ԭ��Bi3+��
������Һ�д���Mn2+�����������ƺ�ɹ۲쵽�������ǣ� ��
�ڷ�Ӧ�����ӷ���ʽΪ�� ��
��6��50��55��ʱ��MnSO4��ĸҺ�м�������NH4HCO3����CO2��MnCO3��������ˮ������Ӧ�Ļ�ѧ����ʽΪ�� ��

��֪��c��Zn2+��=1mol/L����Һ�м���NaOH��Һ��Zn��OH��2�����������ܽ��pH���£�

�Իش��������⣺
��1����ʽп�̵�ع���ʱ������ӦʽΪ��
��2������пˮ��Һ�����ԣ������ӷ���ʽ˵��
��3��д��Zn��OH��2�ĵ��뷽��ʽ
��4������Zn2+������ȫʱ��Һ��ʣ��Zn2+Ũ��Ϊ1��10-5mol/L�����������Ksp[Zn��OH��2]=
��5�����������е�Mn2+���ӿ����������ƣ�NaBiO3��������ˮ�����Լ������ԭ���������Խ����У�Mn2+��������MnO4-��NaBiO3����ԭ��Bi3+��
������Һ�д���Mn2+�����������ƺ�ɹ۲쵽�������ǣ�
�ڷ�Ӧ�����ӷ���ʽΪ��
��6��50��55��ʱ��MnSO4��ĸҺ�м�������NH4HCO3����CO2��MnCO3��������ˮ������Ӧ�Ļ�ѧ����ʽΪ��
���㣺���ʷ�����ᴿ�ķ����ͻ��������ۺ�Ӧ��,�Ʊ�ʵ�鷽�������
ר�⣺ʵ�������
��������1������п�̸ɵ����п������������ʧ���ӷ���������Ӧ��
��2������п����ˮ�������Է�����
��3����������п�����ɺ��ܽ�ͼʾ��֪��������п�����ʺ������������ƣ������������д�����뷽��ʽ��
��4������Ksp=c��Zn2+��?c2��OH-�������㣻
��5�����ݣ���ԭ���������Խ����У�Mn2+��������MnO4-��NaBiO3����ԭ��Bi3+���и�������������ɣ������������Ϊ�Ϻ�ɫ���ݴ�д����Ӧ�����ӷ���ʽ��
��6�����������Ϣ��ԭ���غ�д������ʽ��
��2������п����ˮ�������Է�����
��3����������п�����ɺ��ܽ�ͼʾ��֪��������п�����ʺ������������ƣ������������д�����뷽��ʽ��
��4������Ksp=c��Zn2+��?c2��OH-�������㣻
��5�����ݣ���ԭ���������Խ����У�Mn2+��������MnO4-��NaBiO3����ԭ��Bi3+���и�������������ɣ������������Ϊ�Ϻ�ɫ���ݴ�д����Ӧ�����ӷ���ʽ��
��6�����������Ϣ��ԭ���غ�д������ʽ��
���
�⣺��1������п�̸ɵ����п������������ʧ���ӷ���������Ӧ���缫��ӦʽΪ��Zn-2e-+2OH-=Zn��OH��2��
�ʴ�Ϊ��Zn-2e-+2OH-=Zn��OH��2��
��2��п����ˮ������������п�������ӣ���Һ�����ԣ���Ӧ�����ӷ���ʽΪ��Zn2++2H2O?Zn��OH��2+2H+��
�ʴ�Ϊ��Zn2++2H2O?Zn��OH��2+2H+��
��3����������п�����ɺ��ܽ�ͼʾ��֪��������п���ɷ�����ʽ�����ֿɷ�����ʽ���룬������п�����ʺ������������ƣ������������д�����뷽��ʽΪ��
Zn2++2OH-?Zn��OH��2?ZnO22-+2H+���ʴ�Ϊ��Zn2++2OH-?Zn��OH��2?ZnO22-+2H+��
��4����ȫ����ʱZn2+Ũ��Ϊ1��10-5mol/L����ȫ������pHΪ8����c��H+��=10-8��c��OH-��=
=10-6
Ksp[Zn��OH��2]=c��Zn2+��?c2��OH-��=1��10-5����10-6��2=1��10-17��
�ʴ�Ϊ��1��10-17��
��5����Ӧ���и�������������ɣ�����������ӳ��Ϻ�ɫ���ʿ�����Һ���Ϻ�ɫ��NaBiO3����ǿ�����ԣ���KMnO4������ǿ������Mn2+����Ϊ����������ӣ��÷�Ӧ�����ӷ���ʽΪ��2Mn2++5NaBiO3+14H+=2MnO4-+5Na++5Bi3++7H2O��
�ʴ�Ϊ����Һ��Ϊ�Ϻ�ɫ��2Mn2++5NaBiO3+14H+=2MnO4-+5Na++5Bi3++7H2O��
��6��50��55��ʱ��MnSO4��ĸҺ�м�������NH4HCO3����CO2��MnCO3��������ˮ������Ӧ�ķ���ʽ�ɱ�ʾΪ��MnSO4+2NH4HCO3��MnCO3��+CO2����
����ԭ���غ��֪��Ӧ��������狀�ˮ����ƽ�õ���MnSO4+2NH4HCO3=��NH4��2SO4+MnCO3��+H2O+CO2����
�ʴ�Ϊ��MnSO4+2NH4HCO3=��NH4��2SO4+MnCO3��+CO2��+H2O��
�ʴ�Ϊ��Zn-2e-+2OH-=Zn��OH��2��
��2��п����ˮ������������п�������ӣ���Һ�����ԣ���Ӧ�����ӷ���ʽΪ��Zn2++2H2O?Zn��OH��2+2H+��
�ʴ�Ϊ��Zn2++2H2O?Zn��OH��2+2H+��
��3����������п�����ɺ��ܽ�ͼʾ��֪��������п���ɷ�����ʽ�����ֿɷ�����ʽ���룬������п�����ʺ������������ƣ������������д�����뷽��ʽΪ��
Zn2++2OH-?Zn��OH��2?ZnO22-+2H+���ʴ�Ϊ��Zn2++2OH-?Zn��OH��2?ZnO22-+2H+��
��4����ȫ����ʱZn2+Ũ��Ϊ1��10-5mol/L����ȫ������pHΪ8����c��H+��=10-8��c��OH-��=
| 1��10-14 |
| 10-8 |
Ksp[Zn��OH��2]=c��Zn2+��?c2��OH-��=1��10-5����10-6��2=1��10-17��
�ʴ�Ϊ��1��10-17��
��5����Ӧ���и�������������ɣ�����������ӳ��Ϻ�ɫ���ʿ�����Һ���Ϻ�ɫ��NaBiO3����ǿ�����ԣ���KMnO4������ǿ������Mn2+����Ϊ����������ӣ��÷�Ӧ�����ӷ���ʽΪ��2Mn2++5NaBiO3+14H+=2MnO4-+5Na++5Bi3++7H2O��
�ʴ�Ϊ����Һ��Ϊ�Ϻ�ɫ��2Mn2++5NaBiO3+14H+=2MnO4-+5Na++5Bi3++7H2O��
��6��50��55��ʱ��MnSO4��ĸҺ�м�������NH4HCO3����CO2��MnCO3��������ˮ������Ӧ�ķ���ʽ�ɱ�ʾΪ��MnSO4+2NH4HCO3��MnCO3��+CO2����
����ԭ���غ��֪��Ӧ��������狀�ˮ����ƽ�õ���MnSO4+2NH4HCO3=��NH4��2SO4+MnCO3��+H2O+CO2����
�ʴ�Ϊ��MnSO4+2NH4HCO3=��NH4��2SO4+MnCO3��+CO2��+H2O��
���������⿼���������Ʊ�������������̷����жϣ��������ʺͼ����Ӧ�ã�����ʽ����д���ѵ㣬���뷽��ʽ����дҪ��������Ϣ���н��

��ϰ��ϵ�д�
�����Ŀ
���л�ѧʽ��ָ�����ʵ���Ҫ�ɷֶ�Ӧ��ȷ���ǣ�������
| A��CH4--��Ȼ�� |
| B��CO2--ˮú�� |
| C��CaCO3--ʯ��� |
| D��NaHCO3--�մ�� |
�����������ڴ�������ǣ�������
| A���������� | B��������ϩ |
| C����ά�� | D��Ӳ֬������� |
��25��ʱ���ܱ�������X��Y��Z��������ij�ʼŨ�Ⱥ�ƽ��Ũ�����±�����25��ʱ����ӦX+3Y?2Z��ƽ�ⳣ��Ϊ��������
| ���� | X | Y | Z |
| ��ʼŨ��/mol?L-1 | 0.1 | 0.2 | 0 |
| ƽ��Ũ��/mol?L-1 | 0.05 | 0.05 | 0.1 |
| A��500 |
| B��600 |
| C��1 200 |
| D��1 600 |
�����ǻ�ѧѧϰ���о��ij����ֶΣ����з������ݺͽ��۶���ȷ���ǣ�������
| A��H2O��HCOOH��Cu2��OH��2CO3��������Ԫ�أ����������� |
| B��HCOOH��H2CO3��H2SO4�����о�����������ԭ�ӣ����Ƕ�Ԫ�� |
| C��HF��CH3CH2OH��NaOH��������ˮ�����ǵ���� |
| D��HClO��H2SO4��Ũ����HNO3������ǿ�����ԣ������������� |
�����й�˵����ȷ���� ��������
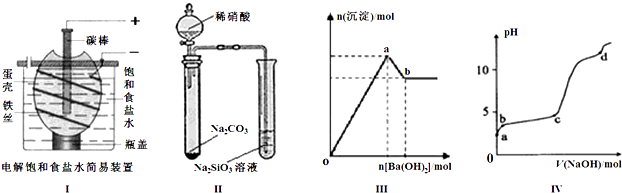

| A����ͼ��װ�õ��һ��ʱ�������������Һ�еμӼ��η�̪����Һ�ʺ�ɫ |
| B����ͼ��װ��ʵ�飬����֤������ǿ����ϵΪ�����̼����� |
| C��ͼ���ʾ����������Һ����μ���Ba��OH��2��Һ�����ɳ��������ʵ�����Ba��OH��2�������ı仯���ߣ���oa�η��������ӷ�ӦΪ�� 2Al3++3SO42-+3Ba2++6OH-=2Al��OH��3��+3BaSO4�� |
| D��ͼ����ʾ������ʱ����1mol?L-1 NaOH��Һ��ε���0.2mol?L-1 Al2��SO4��3��Һ�У�ʵ������ҺpH��NaOH��Һ����仯���ߣ���d��ʱAl��OH��3������ʼ�ܽ� |