��Ŀ����
7�������λ��ͬһ���壬���ǿ����γ�������ɺ��������ƵĻ����һ������þ��Mg2B2O5•H2O����ȡ������Ĺ�������ͼ���£�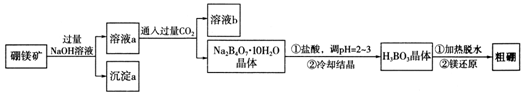
�ش��������⣺
��1��Mg2B2O5•H2O��B�Ļ��ϼ�Ϊ+3��
��2����Һb�����ʵĻ�ѧʽΪNaHCO3��
��3����pH��ֽ����ҺpH�IJ��������ǰ�һС��pH��ֽ���ڱ�������Ƭ���ϣ���պ�д�����Һ�IJ�����������ֽ���в�����ֽ��ɫ�������ɫ���Ƚϣ�
��4��д��Mg2B2O5•H2O�����ᷴӦ�Ļ�ѧ����ʽ��Na2B4O7•10H2O+2HCl=2NaCl+5H2O+4H3BO3��
��5���ƵõĴ�����һ������������BI3��BI3���ȷֽ���Եõ������ĵ������ֽ�0.0200g�����Ƴɵ�BI3��ȫ�ֽ⣬���ɵ�I2��0.3000mo1��•L-1Na2S2O3��Һ�ζ����յ㣬����Na2S2O3��Һ15.00mL������֪��I2+2S2O32-=2I-+S4O62-��
�ٵζ�������ָʾ��ͨ��Ϊ������Һ��
�ڸô�����Ʒ�Ĵ���Ϊ82.5%��
�����ζ�����ʹ��ǰδ��Na2S2O3����Һ��ϴ�������Ʒ�Ĵ��Ƚ�ƫ�ߣ��ƫ�ߡ�����ƫ�͡�����Ӱ�족����
��6���������ͼ��ʽ��ƴӳ���a�л�õ���Mg������ͼ����ʾ���ڼ�ͷ�Ϸ����·���������Լ���ʵ���������

���� ���������λ��ͬһ���壬���ǿ����γ�������ɺ��������ƵĻ��������þ��������������Һ��Ӧ����NaBO2��Mg��OH��2�����ˮ�����˵ó���aΪMg��OH��2���壬��ҺaΪNaBO2��Һ��ͨ������Ķ�����̼���õ�Na2B4O7•10H2O��ΪNaHCO3�����˷��룬����Һb�����ʵĻ�ѧʽΪNaHCO3���������������С�����ᣬ���ϸ��ֽⷴӦ��ǿ���������ԭ������������ܽ�Ƚ�С����Na2B4O7•10H2O���������ᷴӦ�õ����ᣬ���ᾧ����ȷֽ�õ�B2O3�������Mg��ԭ�õ�����
��1�����ݻ��ϼ۴�����Ϊ0����BԪ�ػ��ϼۣ�
��2��������������֪��ͨ�����������̼�������ҺbΪNaHCO3��
��3����һС��pH��ֽ���ڱ�������Ƭ���ϣ���պ�д�����Һ�IJ�����������ֽ���в�����ֽ��ɫ�������ɫ���Ƚϣ�
��4��Na2B4O7•10H2O�����ᷴӦ����ǿ���������ԭ�������Ȼ��ơ�ˮ�����
��5���ٸ��ݵ�����ⵥ����ʾ��ɫ�жϣ�
�ڸ��ݷ�Ӧ����ʽ���ζ����ݼ������������ĺ�����
�����ζ�����ʹ��ǰδ��Na2S2O3����Һ��ϴ������Na2S2O3����Һ���ƫ�ݴ˷�����
��6��Ҫ���õ���Mg���������MgCl2���壬�����Ƚ�����aΪMg��OH��2���壬��Ũ���������ᾧ��MgCl2•6H2O���壬��ΪMgCl2•6H2O������ˮ������������þ��������HCl�������м��ȵõ�MgCl2���壬�ݴ˽��
��� �⣺��1�����ݻ��ϼ۴�����Ϊ0����֪Mg2B2O5•H2O��B�Ļ��ϼ�Ϊ+3���ʴ�Ϊ��+3��
��2��������������֪��ͨ�����������̼�������ҺbΪNaHCO3���ʴ�Ϊ��NaHCO3��
��3����pH��ֽ����ҺpH�IJ��������ǰ�һС��pH��ֽ���ڱ�������Ƭ���ϣ���պ�д�����Һ�IJ�����������ֽ���в�����ֽ��ɫ�������ɫ���Ƚϣ��ʴ�Ϊ����һС��pH��ֽ���ڱ�������Ƭ���ϣ���պ�д�����Һ�IJ�����������ֽ���в�����ֽ��ɫ�������ɫ���Ƚϣ�
��4��Na2B4O7•10H2O�����ᷴӦ����ǿ���������ԭ�������Ȼ��ơ�ˮ�����ᣬ��Ӧ����ʽΪ��Na2B4O7•10H2O+2HCl=2NaCl+5H2O+4H3BO3��
�ʴ�Ϊ��Na2B4O7•10H2O+2HCl=2NaCl+5H2O+4H3BO3��
��5���ٵζ��������еⵥ�ʲ��룬����ʹ�õ�����Һ��Ϊָʾ�����ʴ�Ϊ��������Һ��
����������Ƶ����ʵ���Ϊ��0.3mol/L��0.015L=0.0045mol�����ݹ�ϵʽ��B��BI3��$\frac{3}{2}$I2��3S2O32-��n��B��=$\frac{1}{3}$n��S2O32-��=0.0015mol��
�������Ϊ��11g/mol��0.0015mol=0.0165g����������ĺ���Ϊ��$\frac{0.0165g}{0.02g}$��100%=82.5%��
�ʴ�Ϊ��82.5%��
�����ζ�����ʹ��ǰδ��Na2S2O3����Һ��ϴ������Na2S2O3����Һ���ƫ�����Ը���B��BI3��$\frac{3}{2}$I2��3S2O32-����Ʒ�Ĵ��Ƚ�ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
��6������aΪMg��OH��2���壬��Ũ���������ᾧ��MgCl2•6H2O���壬��ΪMgCl2•6H2O������ˮ������������þ��������HCl�������м��ȵõ�MgCl2���壬����ٵ������MgCl2����õ�þ�����Դӳ���a�л�õ���Mg������ͼΪ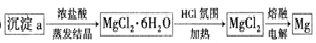 ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼�黯ѧ�Ʊ����������������ԭ���ǽ���ؼ������ضԻ�ѧ����Ŀ��飬��Ҫѧ���߱���ʵ�Ļ�����������õ���������Ŀ�Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д� ��ȩ�ڴ������ڵ������£����Ա��������������ᣮ���ݴ�ԭ�����ʵ���Ƶò����Թ�C���ռ�������������Һ����ͼ��ʾ���Թ�A��װ��40%����ȩˮ��Һ������ͭ��ĩ���Թ�C��װ����������ˮ���ձ�B��װ��ijҺ�壩����֪��60�桫80��ʱ��˫�����������������ɷ�����ȩ��������Ӧ����������ʮ���η�Ӧ������ȫ���й����ʵķе������
��ȩ�ڴ������ڵ������£����Ա��������������ᣮ���ݴ�ԭ�����ʵ���Ƶò����Թ�C���ռ�������������Һ����ͼ��ʾ���Թ�A��װ��40%����ȩˮ��Һ������ͭ��ĩ���Թ�C��װ����������ˮ���ձ�B��װ��ijҺ�壩����֪��60�桫80��ʱ��˫�����������������ɷ�����ȩ��������Ӧ����������ʮ���η�Ӧ������ȫ���й����ʵķе������| ���� | ��ȩ | ���� | ���� | �Ҷ��� | ˮ |
| �е� | 20.8�� | 117.9�� | 290�� | 197.2�� | 100�� |
��1���Թ�A����60�桫80��ʱ��������Ҫ��Ӧ�Ļ�ѧ����ʽΪ��ע����Ӧ������2CH3CHO+O2$\stackrel{60��-80��}{��}$2CH3COOH��
��2����ͼ��ʾ��ʵ��IJ�ͬ�Σ���Ҫ�����¶ȼ����Թ�A�ڵ�λ�ã���ʵ�鿪ʼʱ�¶ȼ�ˮ�����λ��Ӧ���Թ�A�ķ�ӦҺ�У�Ŀ���ǿ��Ʒ�Ӧ�¶�Ϊ60�桫80�棻���Թ�A�ڵ���Ҫ��Ӧ��ɺ�Ӧ��������������¶ȼ�ˮ�����λ��Ӧ�����Թ�A��֧�ܿڴ���
��3���ձ�B��ʢװ��Һ������Ǹ��ͣ�д��һ�ּ��ɣ���
��4����������Թ�C���Ƿ��в������ᣬ���������ṩ��ҩƷ����Ʒ�У�����ʹ�õ���ab��������ĸ��
a��pH��ֽ b��̼�����Ʒ�ĩ
c����ɫʯ����ֽ d��������Һ��
| A�� | 1molˮ������Ϊ18g/mol | |
| B�� | ��״���£�3.01��1023��CO2���ӵ�����Ϊ22g | |
| C�� | ��״���£�1mol�κ��������ԼΪ22.4 L | |
| D�� | ���������Ħ��������64 g |
| A�� | Na2O��CaO��Al2O3������������ | |
| B�� | Һ�岻���磬����Һ���Ƿǵ���� | |
| C�� | 12C��13C�ĺ�������Ų���ʽ��ͬ����ѧ������ͬ | |
| D�� | ֻ�����ۼ�������һ���ǹ��ۻ����� |
| A�� | ������ˮ��Al3++3H2O=Al��OH��3��+3H+ | |
| B�� | �ù�����ˮ���չ�ҵβ���е�SO2��2NH3•H2O+SO2=2NH4++SO32-+H2O | |
| C�� | ��CuCl2��Һ������ʵ�飬���ݷ��⣺CuCl2$\frac{\underline{\;ͨ��\;}}{\;}$Cu2++2C1- | |
| D�� | �ø�����ر���Һ�ζ����2MnO4-+16H++5C2O42-=2Mn2++10CO2��+8H2O |
| A�� | ��֬ | B�� | ��˾ƥ�� | C�� | ������ | D�� | ά���� |
| A�� | �����ЧӦ�����ڼ��������Һ | |
| B�� | �������ӵ�ֱ����1��100 nm֮�� | |
| C�� | ����һ���ǻ���� | |
| D�� | ��FeCl3ϡ��Һ������ˮ�����Ƶ�Fe��OH��3���� |
| A�� | �����£�1 mol•L-1��NH4NO3��Һ�к��е�ԭ�ӵ���ĿΪ2 NA | |
| B�� | 22.4L����ͨ����������������Һ�г�ַ�Ӧ��ת�Ƶĵ�����ΪNA | |
| C�� | ��״���£�5.6 L���Ȼ�̼���еķ�����Ϊ0.25NA | |
| D�� | 4.6gNa��ȫת����Na2O��Na2O2�Ļ���������������������Ϊ0.1NA |
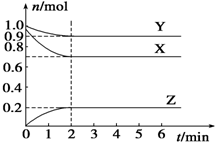 ij�¶�ʱ����һ���ݻ�Ϊ2L���ܱ������У�X��Y��Z���������ʵ����ʵ�����ʱ��ı仯������ͼ��ʾ������ͼ�����ݣ�����д���пհף�
ij�¶�ʱ����һ���ݻ�Ϊ2L���ܱ������У�X��Y��Z���������ʵ����ʵ�����ʱ��ı仯������ͼ��ʾ������ͼ�����ݣ�����д���пհף�