��Ŀ����
10��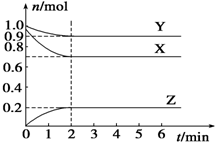 ij�¶�ʱ����һ���ݻ�Ϊ2L���ܱ������У�X��Y��Z���������ʵ����ʵ�����ʱ��ı仯������ͼ��ʾ������ͼ�����ݣ�����д���пհף�
ij�¶�ʱ����һ���ݻ�Ϊ2L���ܱ������У�X��Y��Z���������ʵ����ʵ�����ʱ��ı仯������ͼ��ʾ������ͼ�����ݣ�����д���пհף���1���÷�Ӧ�Ļ�ѧ����ʽΪ3X+Y?2Z��
��2����Ӧ��ʼ��2min������Z�ķ�Ӧ����Ϊ0.05mol•L-1•min-1��
��3����X��Y��Z��Ϊ���壬��Ӧ�ﵽƽ��ʱ��
��ѹǿ�ǿ�ʼʱ��0.9����
������ʱ�������������СΪԭ���� 0.5�����ﵽƽ��ʱ���������¶Ƚ����ͣ����������������Ƚ���������÷�Ӧ������ӦΪ���ȷ�Ӧ������ȡ������ȡ�����
��4����XΪ���塢Y��ZΪ���壬������¶��µ�ƽ�ⳣ��$\frac{1}{45}$��
���� ����ͼ֪�����ŷ�Ӧ���У�X��Y�����ʵ������٣�Z�����ʵ������ӣ�����X��Y�Ƿ�Ӧ���Z�������
��1����ͬʱ���ڣ��μӷ�Ӧ�ĸ����ʵ����ʵ����ı仯��֮�ȵ����������֮�ȣ�
��2����Ӧ��ʼ��2min��v��Z��=$\frac{\frac{��n}{V}}{��t}$��
��3���ٺ��º��������£�����ѹǿ֮�ȵ������ʵ���֮�ȣ�
������ʱ�������������СΪԭ���� 0.5��������ѹǿƽ�������������С�ķ����ƶ����ﵽƽ��ʱ���������¶Ƚ����ͣ�˵���÷�Ӧƽ���ƶ�����������������
��4����ѧƽ�ⳣ��K=$\frac{{c}^{2}��Z��}{c��Y��}$��
��� �⣺����ͼ֪�����ŷ�Ӧ���У�X��Y�����ʵ������٣�Z�����ʵ������ӣ�����X��Y�Ƿ�Ӧ���Z�������
��1����n��X��=��1.0-0.7��mol=0.3mol����n��Y��=��1.0-0.9��mol=0.1mol����n��Z��=��0.2-0��mol=0.2mol����ͬʱ���ڣ��μӷ�Ӧ�ĸ����ʵ����ʵ����ı仯��֮�ȵ����������֮�ȣ�����X��Y��Z�ļ�����֮��=0.3mol��0.1mol��0.2mol=3��1��2����÷�Ӧ����ʽΪ3X+Y?2Z��
�ʴ�Ϊ��3X+Y?2Z��
��2����Ӧ��ʼ��2min��v��Z��=$\frac{\frac{��n}{V}}{��t}$=$\frac{\frac{0.2mol}{2L}}{2min}$=0.05 mol•L-1•min-1���ʴ�Ϊ��0.05 mol•L-1•min-1��
��3���ٿ�ʼʱ���������ʵ���=2.0mol��ƽ��ʱ���������ʵ���=��0.9+0.7+0.2��mol=1.8mol�����º��������£�����ѹǿ֮�ȵ������ʵ���֮�ȣ����Է�Ӧ��ѹǿ�Ƿ�Ӧǰ��$\frac{1.8mol}{2.0mol}$=0.9�����ʴ�Ϊ��0.9��
������ʱ�������������СΪԭ���� 0.5��������ѹǿƽ�������������С�ķ����ƶ����ﵽƽ��ʱ���������¶Ƚ����ͣ�˵���÷�Ӧƽ���ƶ�������������������������Ӧ�����ȷ�Ӧ��
�ʴ�Ϊ�����ȣ�
��4��ƽ��ʱc��Y��=$\frac{0.9mol}{2L}$=0.45mol/L��c��Z��=$\frac{0.2mol}{2L}$=0.1mol/L����ѧƽ�ⳣ��K=$\frac{{c}^{2}��Z��}{c��Y��}$=$\frac{0.1��0.1}{0.45}$=$\frac{1}{45}$
���ʴ�Ϊ��$\frac{1}{45}$��
���� ���⿼�黯ѧƽ����㣬���ؿ���ѧ����������������Ϊ��Ƶ���㣬��ȷ����������֮��Ĺ�ϵ�ǽⱾ��ؼ���ע�⣺��4������㻯ѧƽ�ⳣ��Kʱ���ܰ������壬Ϊ�״��㣮

| A�� | ��������Һ | B�� | ���Ը��������Һ | ||
| C�� | ��ˮ | D�� | ϡ���� |
| A�� | ����Ŀǰ��ֱ�����õ���Դ�����ɻ�ѧ��Ӧ������ | |
| B�� | ú��ʯ�ͣ���Ȼ���ǵ�����������Ҫ�����ֻ�ʯȼ�� | |
| C�� | ���������Դ��ú̿ | |
| D�� | �����˶������ĵ������뻯ѧ��Ӧ�й� |

 ��1����Ӧ3A��g��+B��g���T2C��g�������ֲ�ͬ�������½��з�Ӧ����ͬһʱ���ڣ���õķ�Ӧ�����ò�ͬ�����ʱ�ʾΪ��vA=1 mol/��L•min������vC=0.5mol/��L•min������vB=0.5mol/��L•min������������¸÷�Ӧ�����ɴ�С�Ĺ�ϵ�Ǣۣ��٣��ڣ�������ű�ʾ��
��1����Ӧ3A��g��+B��g���T2C��g�������ֲ�ͬ�������½��з�Ӧ����ͬһʱ���ڣ���õķ�Ӧ�����ò�ͬ�����ʱ�ʾΪ��vA=1 mol/��L•min������vC=0.5mol/��L•min������vB=0.5mol/��L•min������������¸÷�Ӧ�����ɴ�С�Ĺ�ϵ�Ǣۣ��٣��ڣ�������ű�ʾ��