��Ŀ����
14���ҹ������Ƽ���ʳƷҩƷ��CaԪ�غ����IJⶨ����֮һΪ������Na2C2O4�����������Ʒ�е�Ca2+����������ϴ�ӣ�Ȼ������CaC2O4�������ڹ�����ǿ�ᣬ���ʹ����֪Ũ�ȵ�KMnO4��Һͨ���ζ����ⶨ��Һ��Ca2+�ĺ�������Ը�ʵ���еĵζ����̣��ش��������⣺��1��KMnO4��ҺӦ������ʽ�����ʽ����ʽ�����ζ���ʢװ��
��2����д���ζ������з�����Ӧ�����ӷ���ʽ��2MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O
��3���ζ��յ����ɫ�仯Ϊ����Һ������ɫ��Ϊdz��ɫ��
��4��ijͬѧ������ʵ�鷽�������˸Ľ������ڲⶨijƷ�Ƶĸ�Ƭ�еĸ�Ԫ�أ���ҪΪCaCO3����������ʵ��������£�ȡ2.00g��Ʒ������ƿ�У�����ʽ�ζ�������ƿ�ڼ���20.00mLŨ��Ϊ0.10mol•L-1�����ᣨ�������������ַ�Ӧһ��ʱ�䣬�þƾ��ƽ���ƿ��Һ����������ڣ������Ӻ���ȴ�����£�����2��3�����ָʾ������Ũ��Ϊ0.10mol•L-1��NaOH��Һ�ζ����յ㣬����NaOH��Һ8.00mL������ʾ��Ca��OH��2����ˮ��pH�ϵ�ʱ�����������
�ݴ˻ش�
��Ϊʹ�������ԡ����ȷ���ζ������е����ָʾ��Ӧѡ�������Һ���ʯ��������ȡ���̪������
��ʵ������н���ƿ��Һ����е�Ŀ���ǽ��ܽ�����Һ�е�CO2����ϳ�
�����2.00g��Ƭ��CaCO3������Ϊ0.06g��
���� ��1��������ؾ���ǿ�����ԣ������к͵ζ����������жϣ�
��2�������������£�������ؽ�C2O42-����ΪCO2����������ԭΪMnSO4���ݴ�д����Ӧ�����ӷ���ʽ��
��3�����������Һ��������ɫ��Ϊ��ɫ���ڿ�ʼ���뺬C2O42-��Һ��ʱ����ԭ����ɫ��ʧ�����ﵽ�ζ��յ�ʱ���������һ�θ��������Һ��ɫ����ȥ����ҺӦ��������ɫ��Ϊdz��ɫ��
��4��̼��ƺ����ᷴӦ�����Ȼ��ơ�������̼��ˮ�����Ƕ�����̼����ˮ����Ҫ������У������������������Ƶζ��������������Ƶ����ԣ�Ӧѡ����pH�ϵ�ʱ��ɫ��ָʾ�������ݵζ�����������ᣬ���������̼��Ʒ�Ӧ�����ᣬ���ݷ���ʽ����̼��Ƶ�������
��� �⣺��1��������ؾ���ǿ�����ԣ��ḯʴ��Ӧ������ʽ�ζ����У�
�ʴ�Ϊ����ʽ��
��2��������ؾ���ǿ�����ԣ������������½�C2O42-����ΪCO2����������ԭΪMn2+�����ӷ���ʽΪ��2MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O��
�ʴ�Ϊ��2MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O��
��3�����������Һ��������ɫ��Ϊ��ɫ���ڿ�ʼ����C2O42-��ʱ����ԭ����ɫ��ʧ�����ﵽ�ζ��յ�ʱ���������һ�θ��������Һ��ɫ����ȥ����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ��
�ʴ�Ϊ������ɫ��Ϊdz�ϣ�
��4���ٸ��������Ϣ��Ca��OH��2����ˮ��pH�ϵ�ʱ��������������ȱ�ɫ��ΧΪ3.1��4.4�����ϣ�ʯ���ɫ�����ԣ���̪��ɫ��ΧΪ��8.2��10.0��pH�ϸߣ����������Ƴ������ɣ����ŵζ������ü�����ָʾ�����ʴ�Ϊ�����ȣ�
�ڶ�����̼����ˮ��������и�����Һ�ж�����̼�����������̼���������Ʒ�Ӧ������ʴ�Ϊ�����ܽ�����Һ�е�CO2����ϳ���
���к͵ζ���������Ϊ��0.1mol/L��8mL=0.8mmol����̼��Ʒ�Ӧ������Ϊ��0.1mol/L��20mL-0.8mmol=1.2mmol=0.0012mol��
CaCO3+2HCl=CaCl2+H2O+CO2��
100g 2mol
m 0.0012mol
m=$\frac{100g��0.0012mol}{2mol}$=0.06g���ʴ�Ϊ��0.06g��
���� ���⿼���˵ζ�ԭ����Ӧ�ã��е��Ѷȣ�ע�������Ϣ��Ӧ�ã����ݵζ�ԭ�����м���Ҫ��ȷ��Ӧԭ����

| A�� | �е��ʲμӺ͵������ɵĻ�ѧ��Ӧһ����������ԭ��Ӧ | |
| B�� | ������ǿ�ᣬ����������ǿ����� | |
| C�� | ��FeCl3������Һ�μ�NaOH��Һ���Ʊ�Fe��OH��3���� | |
| D�� | ���Ϸ�Ӧ��һ����������ԭ��Ӧ |
| A�� | ���õķ�ɢϵ��������Һ | |
| B�� | �÷�ɢϵ�ܲ��������ЧӦ | |
| C�� | ���õķ�ɢϵ��ˮ�Ƿ�ɢ�� | |
| D�� | �����÷�ɢϵ���ˣ�����ֽ���ܵõ���ɢ�� |
| A�� | ���³�ѹ�£�8 g CH4����4NA����ԭ�� | |
| B�� | 2gD216O�к��е�������Ϊ2NA�� | |
| C�� | 1 mol Cu������FeCl3��Һ��Ӧ��ת��2NA������ | |
| D�� | ͨ��״���£�2.24 L��������0.1NA��N2���� |
ʱ�� ��s��Ũ�� mol•L-1 | 0 | 20 | 40 | 60 | 80 | 100 |
| C��N2O4�� | 0.20 | C1 | 0.10 | C3 | C4 | C5 |
| C��NO2�� | 0.00 | 0.12 | C2 | 0.22 | 0.22 | 0.22 |
��1���÷�Ӧ�Ļ�ѧ����ʽΪ��N2O4?2NO2����ʾC2��C3=C4 ���������=��
��2��20sʱ��������������Ũ��C1=0.14mol/L����0��20s��������������ƽ����Ӧ�ٶ�Ϊ0.003mol/��L•s����

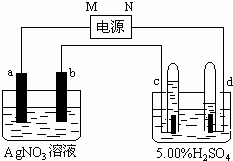 ��ͼ�е缫a��b�ֱ�ΪAg�缫��Pt�缫���缫c��d����ʯī�缫��ͨ��һ��ʱ�����c��d�����Ϲ��ռ���336mL����״̬�����壮��ش��������⣮
��ͼ�е缫a��b�ֱ�ΪAg�缫��Pt�缫���缫c��d����ʯī�缫��ͨ��һ��ʱ�����c��d�����Ϲ��ռ���336mL����״̬�����壮��ش��������⣮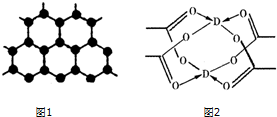 ԭ�����������������������Ԫ��A��B��C��D�ֱ��ڵ�һ���������ڣ�����Aԭ�Ӻ���һ�����ӣ�Bԭ�Ӻ��������6�ֲ�ͬ���˶�״̬��B��C���γ����������ͷ��ӣ�Dԭ����Χ�����Ų�Ϊ3d104s1����ش��������⣺
ԭ�����������������������Ԫ��A��B��C��D�ֱ��ڵ�һ���������ڣ�����Aԭ�Ӻ���һ�����ӣ�Bԭ�Ӻ��������6�ֲ�ͬ���˶�״̬��B��C���γ����������ͷ��ӣ�Dԭ����Χ�����Ų�Ϊ3d104s1����ش��������⣺