��Ŀ����
18��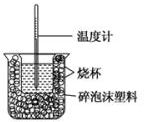 ��֪��ϡ��Һ�У��������кͷ�Ӧ����1molˮʱ�ķ�Ӧ�Ƚ����к��ȣ���������ͼװ�ý����к��ȵIJⶨ����ش��������⣺
��֪��ϡ��Һ�У��������кͷ�Ӧ����1molˮʱ�ķ�Ӧ�Ƚ����к��ȣ���������ͼװ�ý����к��ȵIJⶨ����ش��������⣺��1��ͼʾװ����������Ҫ��ɲ���δ�����������ǻ��β�����������ձ��Ϸ�����ĭ���ϸǣ�
��2���ձ�����������ĭ���ϵ������Ǽ���ʵ������е�������ʧ��
��3��������ʱ�ּ���ע�뷴ӦҺ����õķ�Ӧ����ֵƫС���ƫ��ƫС������Ӱ�족����
��4����һ���������к��Ȳⶨʵ�飬�¶ȼ���ʹ��3�Σ�
��5����ȡ0.5mol/L�������0.55mol/L��NaOH��Һ��50mL����ʵ�飬��������NaOH��Һ��ʼƽ���¶�Ϊt1�棬��Ϸ�Ӧ������¶�Ϊt2�棬����Һ�ܶȾ�Ϊ1g/mL��������Һ�ı�����c=4.18J/g•�森����ʽ�����к��ȣ���H=-$\frac{0.418��{t}_{2}-{t}_{1}��}{0.025}$kJ/mol�����û���
��6����֪��HCl��aq��+NaOH��aq���TNaCl��aq��+H2O��l����H1=a kJ/mol
HCl��aq��+NH3•H2O��aq���TNH4Cl��aq��+H2O��l����H2=b kJ/mol
��NH3•H2O��aq��?NH4+��aq��+OH-��aq����H3=��b-a�� kJ/mol����a��b��ʾ��
���� ��1���������ȼƵĹ������жϸ�װ�õ�ȱ�ٵIJ��֣�
��2���к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�����
��3������ʱ�ּ���ע�뷴ӦҺ����������ɢʧ��
��4���к��Ȳⶨʵ������Ҫ�¶ȼƲ����ᡢ��ͷ�Ӧ�������¶����Σ�
��5���ȸ���Q=m•c•��T���㷴Ӧ�ų���������Ȼ����ݡ�H=-$\frac{Q}{n}$kJ/mol�������Ӧ�ȣ�
��6�����ø�˹���������㷴Ӧ�ʱ䲢��д�Ȼ�ѧ����ʽ��
��� �⣺��1�������ȼƵĹ����֪��װ�õ�ȱ�������ǻ��β�����������ձ��Ϸ�����ĭ���ϸǣ�
�ʴ�Ϊ�����β�����������ձ��Ϸ�����ĭ���ϸǣ�
��2���к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�������С�ձ�֮����������ĭ���ϵ������Ǽ���ʵ������е�������ʧ��
�ʴ�Ϊ������ʵ������е�������ʧ��
��3������ʱ�ּ���ע�뷴ӦҺ����������ɢʧ����õ��к�����ֵ�����С��
�ʴ�Ϊ��ƫС��
��4���к��Ȳⶨʵ������Ҫ�¶ȼƲ����ᡢ��ͷ�Ӧ�������¶����Σ�����������Ҫʹ���¶ȼ�3�Σ�
�ʴ�Ϊ��3��
��5����ʼƽ���¶�Ϊt1�棬��Ϸ�Ӧ������¶�Ϊt2�棬�¶Ȳ�Ϊ����t2-t1���棬0.5mol/L�������0.55mol/L��NaOH��Һ��50mL����ʵ�飬����ˮ�����ʵ���Ϊ0.05L��0.50mol=0.025mol����Һ������Ϊ��100ml��1g/cm3=100g��������0.025molˮ�ų�������ΪQ=m•c•��T=100g��4.18��10-3kJ/��g•�棩����t2-t1���棬����ʵ���õ��к��ȡ�H=-$\frac{100g��4.18��1{0}^{-3}kJ/��g•�棩����t2-t1����}{0.025mol}$=-$\frac{0.418��{t}_{2}-{t}_{1}��}{0.025}$kJ/mol��
�ʴ�Ϊ��-$\frac{0.418��{t}_{2}-{t}_{1}��}{0.025}$��
��6��HCl��aq��+NaOH��aq���TNaCl��aq��+H2O��l����H1=a kJ/mol ��
HCl��aq��+NH3•H2O��aq���TNH4Cl��aq��+H2O��l����H2=b kJ/mol ��
�ɸ�˹���ɢ�-�ٵã�NH3•H2O��aq��?NH4+��aq��+OH-��aq����H3=��b-a��kJ/mol��
�ʴ�Ϊ����b-a����
���� ���⿼���к��ȵIJⶨԭ������㡢��˹���ɵ����ã���Ŀ�Ѷ��еȣ�ע�������к��ȵĸ�����ԭ���ǽ���Ĺؼ���

| A�� | ú̿��������Һ������ȹ��̣��ɻ�������Դ����Ҫ�Ļ���ԭ�� | |
| B�� | ������ʵ�����д�ʩ�Ǽ���������;��֮һ | |
| C�� | �ճ����������Ǵ���ʹ������Ʒ������Ϊ��������������������Ӧ | |
| D�� | ����10�ŷɴ�����̫���ܵ�ذ�ɽ�����ת��Ϊ���ܣ�����ת�������ǵ����� |

| A�� | 0.1mol�ǻ��к���1 mol���� | |
| B�� | ����ͱ�ϩ����Ȼ�ϵ�����1mol�����ȼ�վ�����3molH2O | |
| C�� | CH2Cl2��������ͬ���칹�� | |
| D�� | ͼ���л��1-�������飩��һ�ȴ�����4�� |
| A�� | CaO | B�� | CaCl2 | C�� | NaOH | D�� | C2H6 |
| A�� | �����¶� | B�� | ����������ˮ | ||
| C�� | ��������CuSO4��Һ | D�� | ����Ũ�Ƚϴ������ |
| A�� | ���ȷ�Ӧ�ķ�Ӧ����һ���������ȷ�Ӧ�ķ�Ӧ���� | |
| B�� | ����״̬���ܵ���Ļ�����һ�������Ӽ� | |
| C�� | ����Ӧ��Ũ�ȿɼӿ췴Ӧ���ʣ���˿���Ũ������п��Ӧ������������������ | |
| D�� | �Ƿ��ж���������ǽ������Һ�ı������� |
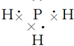 ���ṹʽ
���ṹʽ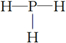 ��
��