��Ŀ����
9����ͼΪ����ʵ��װ�ã�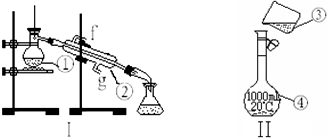
��1��д���������������ƣ�
��������ƿ���������ܣ�
��2�������١����У�ʹ��ʱ�������Ƿ�©ˮ���Тܣ�������ţ�
��3��������װ��I��ȡ����ˮ����ȱ�ٵ������Ǿƾ��ƣ��������������������ʵ�飬��ȴˮ��g�ڽ���
��4����������1.0mol•L-1��NaOH��Һ240mL������װ��II��ijͬѧ���ƴ���Һʱת�Ʋ�����ʾ��ͼ��ͼ������������ֱ���δ�ò�����������δ����250ml����ƿ��
��5��������Һʱ��һ����Է�Ϊ���¼������裺
�ٳ��� �ڼ��� ���ܽ� �ܵ�תҡ�� ��ת�� ��ϴ�� �߶��� ����ȴ
����ȷ�IJ���˳��Ϊ�ڢ٢ۢ�ݢޢߢܣ�
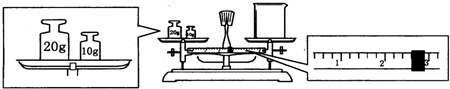
��6��ijͬѧ������һ������NaOH���壬������������ƽ�����ձ�����������ƽƽ����״̬��ͼ���ձ���ʵ������Ϊ27.4g����ͬѧӦ����10.0g NaOH��
��7�������ƹ����У������������������ȷ�ģ����в���������Ũ��ƫ�ߵ��Ǣܣ�
��û��ϴ���ձ��Ͳ����� ��ת����Һʱ������������Һ��������ƿ����
������ƿ�����������������ˮ �ܶ���ʱ���ӿ̶���
�ݶ���ҡ�Ⱥ�����Һ������ڿ̶��ߣ��ٲ�����������ˮ���̶��ߣ�
���� ��1�����������Ľṹ�ص㡢��;�ж������ƣ�
��2���л����Ͳ�����������ʹ��ʱ�������Ƿ�©ˮ��
��3����ȡ����ˮ�Ĺ��̱����þƾ��Ƽ��ȣ�ʵ����������̣��������¿��ǽ�ˮ�ڣ��Ͽ��dz�ˮ�ڣ�
��4����������һ�����ʵ���Ũ�ȵ���Һ�ķ����Ͳ�����Ѱ��װ���еĴ���
��5������Ũ��Һ������ϡ��Һ��ʵ�����������������Ʋ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����
��6��������ƽ��ʹ�÷������������룬���̵������������̵����������������������Ʒ����=��������+�������������λ�÷ŷ����������̵�����=���̵�����+������������е�ʽ���м��㣻����240mL��Һ����Ҫѡ��250mL����ƿ������250mL 1mol/L������������Һ���������Ƶ����ʵ���������������Ƶ�������
��7������c=$\frac{n}{V}$�������������ʵ����ʵ��������Һ�������Ӱ���жϣ�
��� �⣺��1����Ϊ���ڷ���е����ϴ������Һ��ķ�����ѻӷ��Թ�����Һ��ķ����������Ϊ������ƿ����Ϊ��ȴ����ΪҺ��ij������������ܣ�
�ʴ�Ϊ��������ƿ�������ܣ�
��2����Ϊ�ձ�����Ϊ����ƿ������ƿ��ʹ��ʱ��ҡ�ȣ�����ʹ��ǰҪ����Ƿ�©ˮ��������ƿ�������ܲ���Ҫ����Ƿ�©ˮ��
�ʴ�Ϊ���ܣ�
��3����ȡ����ˮ��ʵ����������̣������þƾ��ƣ��������¿��ǽ�ˮ�ڣ��Ͽ��dz�ˮ�ڣ�
�ʴ�Ϊ���ƾ��ƣ�g��
��4������һ�����ʵ���Ũ�ȵ���Һ�DZ����ò�������������ֹҺ���⽦�����ƶ���������Һ��ѡ�����������ƿ��
�ʴ�Ϊ��δ�ò�����������δ����250ml����ƿ��
��5������һ�����ʵ���Ũ�ȵ���Һ����Ϊ�����㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ�������ȷ�IJ���˳��Ϊ���ڢ٢ۢ�ݢޢߢܣ�
�ʴ�Ϊ���ڢ٢ۢ�ݢޢߢܣ�
��6�������̵�����=���̵�����+�����������֪����������=�ձ�����+����������������ձ�����=��������-�������������ձ�����=20g+10g-2.6g=27.4g��ʵ����û��240mL����ƿ��ʵ�������Ƶ���250mL 1mol/L������������Һ����Ҫ�������Ƶ����ʵ���Ϊ��1mol/L��0.25L=0.25mol����Ҫ�������Ƶ�����Ϊ��40g/mol��0.25mol=10.0g��
�ʴ�Ϊ��27.4��10.0g��
��7����û��ϴ���ձ��Ͳ��������������ʵ����ʵ���ƫС����Һ��Ũ��ƫ�ͣ��ʢٲ�ѡ��
��ת����Һʱ������������Һ��������ƿ���棬�������ʵ����ʵ���ƫС����Һ��Ũ��ƫ�ͣ��ʢڲ�ѡ��
������ƿ�����������������ˮ�������ʵ����ʵ�������Һ��������������Ӱ�죬��Һ��Ũ�Ȳ��䣬�ʢ۲�ѡ��
�ܶ���ʱ���ӿ̶��ߣ�������Һ�����ƫС����Һ��Ũ��ƫ�ߣ��ʢ�ѡ��
�ݶ���ҡ�Ⱥ�����Һ������ڿ̶��ߣ��ٲ�����������ˮ���̶��ߣ�������Һ�����ƫ����Һ��Ũ��ƫ�ͣ��ʢݲ�ѡ��
��ѡ���ܣ�
���� ���⿼��������һ�����ʵ���Ũ����Һ�����ƣ���Ϥʵ��ԭ����������ʹ�÷����ǽ���ؼ�����Ŀ�ѶȲ���

 ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�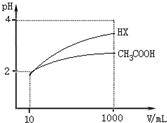 25��ʱ��������ĵ���ƽ�ⳣ�����£�
25��ʱ��������ĵ���ƽ�ⳣ�����£�| ��ѧʽ | CH3COOH | H2CO3 | HClO |
| ����ƽ�ⳣ�� | 1.8��10-5 | K1 4.3��10-7 K2 5.6��10-11 | 3.0��10-8 |
��1��һ������£����¶�����ʱ��Ka�������������С�����䡱����
��2�������������ӽ�����������ɴ�С��˳����a��b��d��c������ţ���
a��CO32-b��ClO- c��CH3COO-d��HCO3-
��3�����з�Ӧ���ܷ�������cd������ţ�
a��CO32-+CH3COOH=CH3COO-+CO2��+H2O
b��ClO-+CH3COOH=CH3COO-+HClO
c��CO32-+2HClO=CO2��+H2O+2ClO-
d.2ClO-+CO2+H2O=CO32-+2HClO
��4��������ˮϡ��0.10mol•L-1�Ĵ��ᣬ���и�ʽ��ʾ����ֵ��ˮ�������Ӷ��������b������ţ���
a.$\frac{c��C{H}_{3}COOH��}{c��{H}^{+}��}$b.$\frac{c��C{H}_{3}CO{O}^{-}��}{c��C{H}_{3}COOH��}$ c.$\frac{c��{H}^{+}��}{{k}_{W}}$ d.$\frac{c��{H}^{+}��}{c��O{H}^{-}��}$
��5�������Ϊ10mL��pH��Ϊ2�Ĵ�����Һ��HX��Һ�ֱ��ˮϡ����1000mL��ϡ������pH�仯��ͼ��ʾ��
��HX�ĵ���ƽ�ⳣ�����ڣ�����ڡ��������ڡ���С�ڡ�����ͬ������ĵ���ƽ�ⳣ����ϡ�ͺ�HX��Һ��ˮ���������c��H+�����ڴ�����Һ��ˮ���������c��H+����������ϡ�ͺ�HX��Һ�е�c��H+��С��CH3COOH��Һ�е�c��H+��������ˮ�ĵ������������������
��6��25��ʱ�������CH3COOH��CH3COONa�Ļ����Һ��pH=6������Һ��c��CH3COO-��-c��Na+��=9.9��10-7 mol•L-1���ȷ��ֵ����
| A�� | ����NaOH��Һʱ���������õ�NaOH�������С�ձ����ܽ⣬δ����ȴ����ת�Ƶ�����ƿ�в����� | |
| B�� | ת�Ƶ�����ƿ�����У���������Һ���� | |
| C�� | ת�ƺ�δϴ��С�ձ��Ͳ����� | |
| D�� | ����ʱ���ӿ̶��� |
| A�� | ������ǰ��ϡ������ϴ����ƿ��δ������ˮϴ�� | |
| B�� | ����ҡ�Ⱥ���Һ����ڿ̶��ߣ��ټ�������ˮ����̶������� | |
| C�� | ϴ����Ͳ������ϴ��Һת������ƿ | |
| D�� | ����ʱ���Ӷ����� |

 ��
��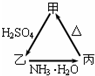 �ס��ҡ�����������֮��������ͼ��ʾ��ת����ϵ��
�ס��ҡ�����������֮��������ͼ��ʾ��ת����ϵ��