��Ŀ����
1�� ������Ҫ�ķǽ���Ԫ�أ������γɶ��ֻ�����ش��������⣺
������Ҫ�ķǽ���Ԫ�أ������γɶ��ֻ�����ش��������⣺��1����̬��ԭ�ӵĵ����Ų�ʽ��1s22s22p3��C��N��O����Ԫ�ص�һ�����ܴӴ�С��˳����N��O��C��
��2���£�N2H4�����ӿ���ΪNH3�����е�һ����ԭ�ӱ�-NH2��������ȡ���γɵ���һ�ֵ����⻯�
��NH3���ӵĿռ乹���������ͣ�N2H4�����е�ԭ�ӹ�����ӻ�������sp3��
���¿��������ȼ�ϣ�ȼ��ʱ�����ķ�Ӧ�ǣ�N2O4��1��+2N2H4��1���T3N2��g��+4H2O��g����H=-1038.7kJ•mol-1�����÷�Ӧ����4molN-H�����ѣ����γɵĦм���3mol��
�����������ᷴӦ����N2H6SO4��N2H6SO4�����������������ͬ����N2H6SO4�ľ����ڲ�����d�����ţ�
a�����Ӽ�b�����ۼ�c����λ�� d�����»���
��3����CH3��3NH+��AlCl4-�γ�����Һ�壮����Һ����������������ɣ��۵����100�棬��ӷ���һ����л��ܼ�С ���С������������b������ţ���
a����ȼ�� b������ɫ���ܼ�c�����ϲ��� d�����Ȳ���
��4��X+�����е������ó���K��L��M�������Ӳ㣬����N3-�γɵľ���ṹ��ͼ��ʾ��X��Ԫ�ط�����Cu ����ͬһ��N3-������X+��6����
���� ��1��Nԭ�Ӻ�����7�����ӣ���ԭ�ӵĵ����Ų�ʽ1s22s22p3��ͬһ����Ԫ�صĵ�һ����������ԭ�����������������������ƣ�����IIA��͵�VA��Ԫ�صĵ�һ�����ܴ�������Ԫ�أ�
��2���ٸ��ݼ۲���ӶԻ�������ȷ�����ӿռ乹�ͺ�ԭ�ӵ��ӻ���ʽ��
�ڷ�Ӧ����4mol N-H�����ѣ���μӷ�Ӧ��N2H4Ϊ1mol�����ݷ���ʽ��������N2�����ʵ������������ӽṹʽΪN��N�������к���1���Ҽ���2���м���
�۸�������茶����д��ڵĻ�ѧ���жϣ�
��3������Һ���е������������Ӽ�����������ǿ������ͷ��Ӽ���������
��4�������ж�XΪCuԪ�أ����þ�̯�����㣮
��� �⣺��1��Nԭ�Ӻ�����7�����ӣ���ԭ�ӵĵ����Ų�ʽ1s22s22p3��C��N��O����ͬһ����Ԫ����ԭ���������μ�С��ͬһ����Ԫ�صĵ�һ����������ԭ������������������ڢ�A��Ĵ��ڵڢ�A��ģ��������һ�����ܴ�С˳����N��O��C��
�ʴ�Ϊ��1s22s22p3��N��O��C��
��2����NH3�����е�ԭ�Ӻ���3�����ۼ���һ���µ��Ӷԣ����Կռ乹���������ͣ�N2H4�����е�ԭ�ӵļ۲���Ӷ�=3+1=4������һ���µ��Ӷԣ�Nԭ�ӹ�����ӻ�������sp3��
�ʴ�Ϊ�������ͣ�sp3��
�ڷ�Ӧ����4mol N-H�����ѣ���μӷ�Ӧ��N2H4Ϊ1mol������2N2H4��l��+N2O4��l���T3N2��g��+4H2O��g����֪����N2�����ʵ���Ϊ1mol��$\frac{3}{2}$=1.5mol���������ӽṹʽΪN��N�������к���1���Ҽ���2���м������γɵĦм�Ϊ1.5mol��2=3mol��
�ʴ�Ϊ��3��
������������ӻ����������д������Ӽ����ۼ���N2H6SO4�����������������ͬ������N2H6SO4�ľ����ڴ������Ӽ����ۼ��������������ͷ��»�����
�ʴ�Ϊ��d��
��3������Һ���е������������Ӽ�����������ǿ������ͷ��Ӽ���������������ӷ��Ծ�С��������Ⱦ�������ǡ���ɫ���ܼ���
�ʴ�Ϊ��С��b��
��4��X+��������=2+8+18=28������XΪ29��CuԪ�أ���ͼ�Ͽ����١�࣬���X3N֪����ΪN3-���Զ����ϵġ�Ϊ���ģ����������X+��3��������һ����������ά�ռ���8�������������壬��ÿ��N3-��Χ��X+��8��3��$\frac{1}{4}$=6��������ԭ��Ϊ4�����������ã�����ÿ��ԭ���ڴ˾�����Ϊ$\frac{1}{4}$�ݣ���
�ʴ�Ϊ��Cu��6��
���� ���⿼���˷��ӿռ乹�͵��жϡ�ԭ�ӵ��ӻ���ʽ����ѧ���ļ��㡢�����д��ڵĻ�ѧ����֪ʶ�㣬�ѶȲ���ע����ӿռ乹�͵��жϼ�ԭ���ӻ���ʽ���ж��Ǹ߿����ȵ㣮

 ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д� Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
Happy holiday���ּ��������ҵ�㶫���������ϵ�д�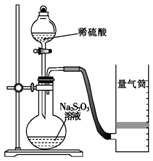 ������ijͬѧ�ⶨ��ѧ��Ӧ���ʲ�̽����Ӱ�����ص�ʵ�飮
������ijͬѧ�ⶨ��ѧ��Ӧ���ʲ�̽����Ӱ�����ص�ʵ�飮�ⶨ��ѧ��Ӧ����
��ͬѧ������ͼװ�òⶨ��ѧ��Ӧ���ʣ�
����֪��S2O32-+2H+=H2O+S��+SO2����
��1������ͼ��ʾ��ʵ����Ʒ�������⣬����Ҫ��һ��ʵ�������������
��2������2minʱ�ռ���224mL��������ɱ�״�������壬�ɼ������2min��H+�ķ�Ӧ���ʣ����òⶨֵ��ʵ��ֵƫС����ԭ����SO2�Ჿ������ˮ�����������SO2���ƫС��
��3���Լ����ⶨ�÷�Ӧ�Ļ�ѧ��Ӧ���ʵ������������ⶨһ��ʱ�����������ʵ�������ⶨһ��ʱ������ҺH+Ũ�ȵı仯�ȣ�дһ�֣���
��Ϊ̽�ֻ�ѧ��Ӧ���ʵ�Ӱ�����أ���Ƶ�ʵ�鷽�����������֪ I2+2S2O32-=S4O62-+2I-������Na2S2O3��Һ��������
| ʵ����� | ���V/mL | ʱ��/s | |||
| Na2S2O3��Һ | ������Һ | ��ˮ | ˮ | ||
| �� | 10.0 | 2.0 | 4.0 | 0.0 | t1 |
| �� | 8.0 | 2.0 | 4.0 | 2.0 | t2 |
| �� | 6.0 | 2.0 | 4.0 | Vx | t3 |
| A�� | AlCl3��Һ�м��������İ�ˮ��Al3++3OH-�TAl��OH��3�� | |
| B�� | �������������ռ���Һ��Ӧ��Cl2+2OH-�TCl-+ClO-+H2O | |
| C�� | ͭ��ϡ���ᷴӦ��Cu+4H++2NO3-�TCu2++2NO2��+2H2O | |
| D�� | ������SO2ͨ��NaOH�У�SO2+2OH-�TSO32-+H2O |
| A�� | ��������ӵĽṹʽ�� | |
| B�� | ������Ϊ37����ԭ�ӣ�${\;}_{37}^{17}$Cl | |
| C�� | NH4Cl�ĵ���ʽ�� | |
| D�� | ԭ�Ӻ�����10�����ӵ���ԭ�ӣ�${\;}_{8}^{18}$O |
| A�� | S��ȼ����Ϊ297.23 kJ/mol | |
| B�� | �γ�1 mol SO2�Ļ�ѧ�����ͷŵ����������ڶ���1mol S��s����1mol O2��g���Ļ�ѧ�������յ������� | |
| C�� | S��g��+O2��g��=SO2��g���ų�������С��297.23 kJ | |
| D�� | 1mol SO2���������� 1mol S��s����1mol O2��g���������� |

 ��R��R�䡢R�������������⣩
��R��R�䡢R�������������⣩ ��
�� ��
�� ��
�� ��
��

 ���ýṹ��ʽ��ʾ�л���ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ��������
���ýṹ��ʽ��ʾ�л���ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ��������