��Ŀ����
7����Ԫ�����γɶ��ֶ����Ļ������ش���1��298Kʱ����2L�̶�������ܱ������У��������淴Ӧ��2NO2��g��?N2O4��g����H=-a kJ/mol ��a��0����N2O4�����ʵ���Ũ����ʱ��仯��ͼ1����ƽ��ʱ��N2O4��Ũ��ΪNO2��2�����ش��������⣺
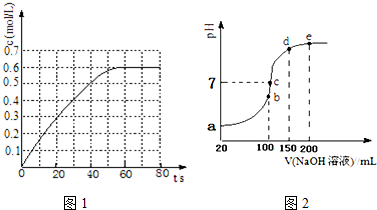
��298kʱ���÷�Ӧ��ƽ�ⳣ��Ϊ6.67L•mol-1����ȷ��0.01����
������������������жϸ÷�Ӧ�Ƿ���ƽ��״̬����A��
A�����������ܶȱ��ֲ���
B������������ɫ���ٱ仯
C��������������ѹǿ���ֲ���
������Ӧ��398K���У�ijʱ�̲��n��NO2��=0.6mol��n��N2O4��=1.2mol�����ʱv����v�������������������=������
��2�����������£���100mL 0.1mol•L-1NH4HSO4��Һ�еμ�0.1mol•L-1NaOH��Һ���õ�����ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ2��ʾ���Է���ͼ��a��b��c��d��e����㣨������������ڶ�����������ȫ�ģ���
��a����Һ��pH��1�����������������=������
��b����Һ�з���ˮ�ⷴӦ��������NH4+��
��c����Һ�и�����Ũ���ɴ�С������˳��Ϊc��Na+����c��SO42-����c��NH4+����c��OH-��=c��H+����
��d��e���Ӧ��Һ�У�ˮ����̶ȴ�С��ϵ��d��e�����������������=������
���� ��1������ͼ��֪N2O4��ƽ��Ũ��Ϊ0.6mol/L���ﵽƽ��ʱ��N2O4��Ũ��ΪNO2��2������NO2��ƽ��Ũ��Ϊ0.3mol/L������ƽ�ⳣ������ʽ���㣻
��A��������������䣬�ݻ��㶨�����������ܶ�Ϊһ��ֵ��
B������������ɫ���䣬˵��NO2��Ũ�ȱ��ֺ㶨��
C����Ӧ2NO2��g��?N2O4��g����ѹ�㶨ʱ�������淴Ӧ������ȣ�
�۷�ӦΪ���ȷ�Ӧ�������¶ȣ�Kֵ��С�������֪��ʱ��Ũ����Q=K��298K����K��398K������Ӧ���淴Ӧ�����ƶ������V��������V���棩��
��2���ٳ��������£���100mL 0.1mol•L-1NH4HSO4��Һ�еμ�0.1mol•L-1NaOH��Һ�����������Һ�����ԣ�笠�����ˮ�������ԣ�
��a��b��c��d��e����㣬���ݷ�Ӧ���Ĺ�ϵ��b��ǡ��������H+����Һ��ֻ�У�NH4��2SO4��Na2SO4��
��c����Һ�����ԣ�����Һ���У�NH4��2SO4��Na2SO4��NH3•H2O���ֳɷ֣�
�ܸ��ݼ�����Һ�У�����������Ũ��Խ��ˮ�ĵ���̶�ԽС���з�����
��� �⣺��1������ͼ��֪N2O4��ƽ��Ũ��Ϊ0.6mol/L���ﵽƽ��ʱ��N2O4��Ũ��ΪNO2��2������NO2��ƽ��Ũ��Ϊ0.3mol/L����K=$\frac{c��{N}_{2}{O}_{4}��}{{c}^{2}��N{O}_{2}��}$=$\frac{0.6}{0��{3}^{2}}$=6.67��
�ʴ�Ϊ��6.67��
��A����Ӧ���������ȫ����̬���ʣ�������������䣬�ݻ�Ϊ2L���ֺ㶨���ɦ�=$\frac{m}{V}$��֪������ܶ�Ϊһ��ֵ�����������ܶȱ��ֲ��䲻һ������ƽ��״̬����A��ȷ��
B������������ɫ����˵��NO2��Ũ�Ȳ��䣬˵����Ӧ���ڻ�ѧƽ��״̬����B����
C����Ӧ2NO2��g��?N2O4��g����һ�����������С�ķ�Ӧ����ѹ�㶨ʱ�������淴Ӧ������ȣ�˵����Ӧ���ڻ�ѧƽ��״̬����C����
�ʴ�Ϊ��A��
�۷�ӦΪ���ȷ�Ӧ�������¶ȣ�Kֵ��С���ܱ����������Ϊ2L����˵�N2O4��Ũ��Ϊ0.6mol/L��NO2��Ũ��Ϊ0.3mol/L��Ũ����Q�T$\frac{c��{N}_{2}{O}_{4}��}{{c}^{2}��N{O}_{2}��}$=$\frac{0.6}{0��{3}^{2}}$=6.67=K��298K����K��398K������Ӧ���淴Ӧ�����ƶ����ʣ�V��������V���棩��
�ʴ�Ϊ������
��2�������������Һ�����ԣ�笠�����ˮ�������ԣ�0.1mol•L-1NH4HSO4��Һ�� ������Ũ�ȴ���0.1mol/L����ҺPH��1��
�ʴ�Ϊ������
��a��b��c��d��e����㣬���ݷ�Ӧ���Ĺ�ϵ��b��ǡ��������H+����Һ��ֻ�У�NH4��2SO4��Na2SO4����Һ�з���ˮ�ⷴӦ��������NH4+��
�ʴ�Ϊ��NH4+��
��c����Һ�����ԣ�����Һ���У�NH4��2SO4��Na2SO4��NH3•H2O���ֳɷ֣�b��ʱc��Na+��=c��SO42-����c��ʱc��Na+����c��SO42-��������NԪ����SԪ�صĹ�ϵ�����Եó�c��SO42-����c��NH4+������Һ������Ũ�ȴ�СΪ��c��Na+����c��SO42-����c��NH4+����c��OH-��=c��H+����
�ʴ�Ϊ��c��Na+����c��SO42-����c��NH4+����c��OH-��=c��H+����
��d��e��Һ��Ϊ������Һ����Һ�е�����������������ˮ�ĵ��룬������������Һ���Խ��ˮ�ĵ������ԽС����d��ˮ�ĵ���̶ȴ���e��ˮ�ĵ��룬
�ʴ�Ϊ������
���� ���⿼��ƽ�ⳣ���ļ��㡢ƽ����ƶ�������Ũ�ȴ�С�Ƚϡ�����ˮ���֪ʶ�㣬��Ŀ�Ѷ��еȣ�����֪ʶ��϶࣬�ۺ��Խ�ǿ����ֿ�����ѧ���ķ�������������������Ũ�ȴ�С�Ƚϳ�������ˮ�⡢������ʵĵ������Ͽ��飬ȷ������Ũ�ȴ�СʱҪ��ϵ���غ㡢�����غ����������

| A�� | ������Ӧ������������ԭ��Ӧ�����������ӷ�Ӧ | |
| B�� | ����1 mol Cl2ת�Ƶ�������Ϊ2NA | |
| C�� | ����Ũ��Խ��Cl-�Ļ�ԭ��Խǿ | |
| D�� | �����ԣ�KMnO4��K2Cr2O7��Cl2��MnO2 |
| A�� | �� | B�� | ��ʯ�� | C�� | ��ˮ����ͭ | D�� | ��ʯ |
| A�� | 2 | B�� | 3 | C�� | 4 | D�� | 6 |

| A�� | ���ձ�a�м�������K3[Fe��CN��6]��Һ��û����ɫ�������� | |
| B�� | �ձ�b�з�����ӦΪZn+2e-=Zn2+ | |
| C�� | ���Ӵ�Zn������������Fe���������Żص�Zn�� | |
| D�� | �ձ�a�з�����ӦO2+4H++4e-=2H2O����ҺpH���� |
| A�� | ��Һ��ˮ�������c��H+��=10-10 mol•L-1 | |
| B�� | ��Һ��c��H+��+c��A-��=0.1 mol•L-1 | |
| C�� | ��0.05 mol•L-1 NaOH��Һ�������ϣ�ˮ�ĵ���ƽ���������ƶ� | |
| D�� | ������Һ�м���һ����NaA������ˮϡ�ͣ���Һ��c��OH-�������� |
| A�� | ���ȶ��ԣ�NaHCO3��Na2CO3 | |
| B�� | �������������Ƽ������� | |
| C�� | ����Һ������ͬʱ��Ũ�ȣ�Na2CO3��NaHCO3 | |
| D�� | ��ͬŨ�ȵ�������Һ��Ӧ�ų����ݵĿ����̶ȣ�NaHCO3��Na2CO3 |