��Ŀ����
4���״���һ�ֿ�������Դ�����ճ����������Ź㷺��Ӧ�ã���ҵ����CO����ȼ�ϼ״����磺CO��g��+2H2��g��?CH3OH��g����ͼ1��ʾ��Ӧ�������ı仯��ͼ2��ʾһ���¶��£������Ϊ2L���ܱ������м���4mol H2��һ������CO��CO��CH3OH��g����Ũ����ʱ��仯��
��ش��������⣺
��1��ͼ1��ʾʹ�ú�δʹ�ô���ʱ��Ӧ���̺������Ķ�Ӧ��ϵ�������йش�����˵������ȷ��A
A�����ͷ��ӵ����� B�������˻������
C������˻���Ӱٷ��� D�������˵�λ����ڵĻ������
��2���ӷ�Ӧ��ʼ������ƽ�⣬�ù������ͷ�136.5 kJ������
��3����T2���£���2molCO��6molH2����2L���ܱ������У��ﵽƽ��״̬ʱ�����c��CO��=0.2mol•L-1����CO��ת����Ϊ80%��
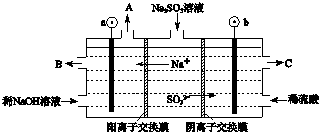
��4����ͼ3Ϊ���ñʼDZ��������ü״����ӽ���Ĥȼ�ϵ�صĽṹʾ��ͼ�磮����ܷ�ӦΪ2CH3OH+3O2?2CO2+4H2O���ڵ��Ե�ʹ�ù����У���ص��¶�������Ϊ����ԭ������ߣ��¶����߲����ڣ���������ڡ������ڡ�����ؽ���ѧ��ת��Ϊ���ܣ���װ����a�����a����b����Ϊ��صĸ������õ缫��ӦʽΪCH3OH+H2O-6e-=CO2��+6H+��
���� ��1��ʹ�ô���ͨ���ı䷴Ӧ�����̣����ͷ�Ӧ�Ļ�ܣ������˻������Ŀ����ٷֺ������Ӷ��ӿ컯ѧ��Ӧ���ʣ�
��2������ͼ1д����Ӧ���Ȼ�ѧ����ʽ�������Ȼ�ѧ����ʽ��������0.75mol/L��2L=1.5mol�Ҵ�ʱ�ų���������
��3�����ݡ�����������������ʼ����ƽ��������ת�������Ӷ�����ת���ʣ�
��4��2CH3OH+3O2?2CO2+4H2OΪ���ȷ�Ӧ�������¶�ƽ���������ƶ����ݴ��ж�K�仯�����������ӵ��ƶ������ж�ȼ�ϵ�صĵ缫����������������Ӧ������������ԭ��Ӧ��
��� �⣺��1��ʹ�ô���ͨ���ı䷴Ӧ�����̣����ͷ�Ӧ�Ļ�ܣ������˻������Ŀ����ٷֺ������ӿ췴Ӧ���ʣ�
��ѡA��
��2������ͼ1д����Ӧ���Ȼ�ѧ����ʽ��CO��g��+2H2��g��?CH3OH��g����H=-91KJ/mol��
����ͼ2��֪��Ӧ�ﵽƽ��ʱ�����Ҵ�1.5mol����Ӧ��Ϊ��-91KJ/mol��1.5mol=-136.5KJ/mol���ų�����136.5KJ��
�ʴ�Ϊ��136.5��
��3����ͼ2��֪����Ӧ�м�С��CO��Ũ��Ϊ1mol/L-0.25mol/L=0.75mol/L��10minʱ�ﵽƽ�⣬
���ݷ���ʽ��
CO��g��+2H2��g��?CH3OH��g��
��ʼ 1mol/L 2mol/L 0
ת�� 0.75mol/L 1.5mol/L 0.75mol/L
ƽ�� 0.25mol/L 0.5mol/L 0.75mol/L
CO��ת����Ϊ$\frac{0.8mol/L}{1mol/L}$��100%=80%��
�ʴ�Ϊ��80%��
��4��2CH3OH+3O2?2CO2+4H2OΪ���ȷ�Ӧ�������¶�ƽ���������ƶ����÷�Ӧ��ƽ�ⳣ��K����С�������õ�ؽ���ѧ��ת��Ϊ���ܣ����������������֪b�缫Ϊ������a�缫Ϊ��������������������Ӧ���״���b��ʧȥ���ӷ���������Ӧ���ɶ�����̼�������ӣ��缫��ӦΪ��CH3OH+H2O-6e-=CO2��+6H+��
�ʴ�Ϊ�������ڣ�a��CH3OH+H2O-6e-=CO2��+6H+��
���� �����漰�绯ѧ���Ȼ�ѧ�Լ���ѧ��Ӧ���ʺͻ�ѧƽ����ۺ�֪ʶ�Ŀ��飬ע�⻯ѧƽ�ⳣ���ļ��㼰�����壬�缫����ʽ��дʱע��������Һ�Ƿ���뷴Ӧ����Ŀ�Ѷ��еȣ�

 ��������ϵ�д�
��������ϵ�д� ��ӡ�Ļ���ʱ����ϵ�д�
��ӡ�Ļ���ʱ����ϵ�д� ��ѧ�����ϵ�д�
��ѧ�����ϵ�д�| A�� | C2H4 | B�� | C6H6 | C�� | BF3 | D�� | NH3 |
| A�� | x=4 | B�� | B��ת����Ϊ60% | ||
| C�� | A��ƽ��Ũ����2.8mol/L | D�� | ƽ��ʱ�����ѹǿ��ԭ����0.94�� |
| A�� | 64gSO2������ԭ����Ϊ2NA | |
| B�� | NA�������Ӻ�NA������ӵ������ȵ���16��1 | |
| C�� | 28g����������ԭ����ĿΪNA | |
| D�� | ��״���£�22.4L��ˮ���� NA��ˮ���� |
| A�� | ��Һ����ȡ������ | B�� | ��ȡ������Һ | C�� | ��Һ��������ȡ | D�� | ������ȡ����Һ |
 ��Ԫ�ؿ����γ�HClO��HClO2��HClO3��HClO4���ֺ����ᣮ
��Ԫ�ؿ����γ�HClO��HClO2��HClO3��HClO4���ֺ����ᣮ