��Ŀ����
��1���л���Aȼ�պ�ֻ������CO2��H2O��g�������ǵ����ʵ���֮��Ϊ
=1��2�����л���Ľṹ��ʽΪ ��
��2����ϵͳ��������д�����л�������ƣ�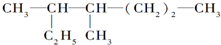 ��������
��������
��3���л���X�ļ���ʽ��ͼ��ʾ��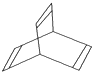
��X�ķ���ʽΪ ��
���л���Y��X��ͬ���칹�壬�����ڷ����廯�����Y�Ľṹ��ʽ�� ��Y��������ˮ��Ӧ����Z���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��Z��NaOH���Ҵ���Һ���ȵõ�ֻ��������Ԫ�ص��л���˷�Ӧ�������� ����Ӧ����ʽΪ ��
| n(CO2) |
| n(H2O) |
��2����ϵͳ��������д�����л�������ƣ�
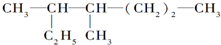 ��������
����������3���л���X�ļ���ʽ��ͼ��ʾ��

��X�ķ���ʽΪ
���л���Y��X��ͬ���칹�壬�����ڷ����廯�����Y�Ľṹ��ʽ��
��Z��NaOH���Ҵ���Һ���ȵõ�ֻ��������Ԫ�ص��л���˷�Ӧ��������
���㣺�л�����ƶ�,�л�����������
ר�⣺�л���Ļ�ѧ���ʼ��ƶ�,�л���������ͨʽ��Ӧ�ù���
��������1���л���Aȼ�պ�ֻ����CO2��H2O��g������A��һ������C��HԪ�أ����ܺ���OԪ�أ����ʵ���֮��Ϊ
=1��2��A��C��Hԭ����֮��Ϊ1��4���ݴ�ȷ��A���ܵĽṹ��ʽ��
��2�����л���Ϊ����������������ϵͳ����������������
��3���ٸ����л���Ľṹ��ʽ�ж��л���ķ���ʽ�� �ķ���ʽΪC8H8��
�ķ���ʽΪC8H8��
��Y�к��б�����Y�Ľṹ��ʽ ������̼̼˫�����ɷ����ӳɷ�Ӧ��д��
������̼̼˫�����ɷ����ӳɷ�Ӧ��д�� ����ˮ��Ӧ�Ļ�ѧ����ʽ��
����ˮ��Ӧ�Ļ�ѧ����ʽ��
��Z�IJ�������������ԭ�ӣ���NaOH���Ҵ���Һ���ȿ��Է�����ȥ��Ӧ���ɱ���Ȳ���ݴ�д����Ӧ�Ļ�ѧ����ʽ��
| n(CO2) |
| n(H2O) |
��2�����л���Ϊ����������������ϵͳ����������������
��3���ٸ����л���Ľṹ��ʽ�ж��л���ķ���ʽ��
 �ķ���ʽΪC8H8��
�ķ���ʽΪC8H8����Y�к��б�����Y�Ľṹ��ʽ
 ������̼̼˫�����ɷ����ӳɷ�Ӧ��д��
������̼̼˫�����ɷ����ӳɷ�Ӧ��д�� ����ˮ��Ӧ�Ļ�ѧ����ʽ��
����ˮ��Ӧ�Ļ�ѧ����ʽ����Z�IJ�������������ԭ�ӣ���NaOH���Ҵ���Һ���ȿ��Է�����ȥ��Ӧ���ɱ���Ȳ���ݴ�д����Ӧ�Ļ�ѧ����ʽ��
���
�⣺��1�������л���Aȼ�պ�ֻ����CO2��H2O��g����֪��A������һ������C��HԪ�أ����ܺ���OԪ�أ���ȼ�ղ���
=1��2����A��C��Hԭ����֮��Ϊ1��4����AΪ������AΪ���飬��ṹ��ʽΪ��CH4����A�к�����ԭ�ӣ���AΪ�״�����ṹ��ʽΪ��CH3OH��
�ʴ�Ϊ��CH4��CH3OH��
��2��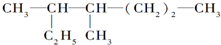 Ϊ�������̼������7��C������Ϊ���飬��ŴӾ�����������˿�ʼ����3��4��C������1���������л�������Ϊ��2��3-�������飬
Ϊ�������̼������7��C������Ϊ���飬��ŴӾ�����������˿�ʼ����3��4��C������1���������л�������Ϊ��2��3-�������飬
�ʴ�Ϊ��2��3-�������飻
��3�����ɽṹ��ʽ ��֪�����л���X�ķ���ʽΪ��C8H8��
��֪�����л���X�ķ���ʽΪ��C8H8��
�ʴ�Ϊ��C8H8��
���л���Y��X��ͬ���칹�壬�����ڷ����廯�����Y�����к��б������Ҳ�������̼̼˫����Y�Ľṹ��ʽΪ�� ��
��
 ����̼̼˫����������ˮ�����ӳɷ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ��
����̼̼˫����������ˮ�����ӳɷ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
�� ��
��
��Z�Ľṹ��ʽΪ�� ��Z��NaOH���Ҵ���Һ���ȵõ�ֻ��������Ԫ�ص��л���ò���Ϊ����Ȳ����Ӧ����ʽΪ��
��Z��NaOH���Ҵ���Һ���ȵõ�ֻ��������Ԫ�ص��л���ò���Ϊ����Ȳ����Ӧ����ʽΪ�� +2NaOH
+2NaOH
 +2NaBr���÷�ӦΪ��ȥ��Ӧ��
+2NaBr���÷�ӦΪ��ȥ��Ӧ��
�ʴ�Ϊ����ȥ��Ӧ�� +2NaOH
+2NaOH
 +2NaBr��
+2NaBr��
| n(CO2) |
| n(H2O) |
�ʴ�Ϊ��CH4��CH3OH��
��2��
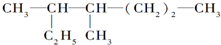 Ϊ�������̼������7��C������Ϊ���飬��ŴӾ�����������˿�ʼ����3��4��C������1���������л�������Ϊ��2��3-�������飬
Ϊ�������̼������7��C������Ϊ���飬��ŴӾ�����������˿�ʼ����3��4��C������1���������л�������Ϊ��2��3-�������飬�ʴ�Ϊ��2��3-�������飻
��3�����ɽṹ��ʽ
 ��֪�����л���X�ķ���ʽΪ��C8H8��
��֪�����л���X�ķ���ʽΪ��C8H8���ʴ�Ϊ��C8H8��
���л���Y��X��ͬ���칹�壬�����ڷ����廯�����Y�����к��б������Ҳ�������̼̼˫����Y�Ľṹ��ʽΪ��
 ��
�� ����̼̼˫����������ˮ�����ӳɷ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ��
����̼̼˫����������ˮ�����ӳɷ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ�� ��
���ʴ�Ϊ��
 ��
�� ��
����Z�Ľṹ��ʽΪ��
 ��Z��NaOH���Ҵ���Һ���ȵõ�ֻ��������Ԫ�ص��л���ò���Ϊ����Ȳ����Ӧ����ʽΪ��
��Z��NaOH���Ҵ���Һ���ȵõ�ֻ��������Ԫ�ص��л���ò���Ϊ����Ȳ����Ӧ����ʽΪ�� +2NaOH
+2NaOH| �Ҵ� |
| �� |
 +2NaBr���÷�ӦΪ��ȥ��Ӧ��
+2NaBr���÷�ӦΪ��ȥ��Ӧ���ʴ�Ϊ����ȥ��Ӧ��
 +2NaOH
+2NaOH| �Ҵ� |
| �� |
 +2NaBr��
+2NaBr��
���������⿼�����л���ṹ�����ʡ��л����������л���ṹ��ʽ��ȷ������Ŀ�Ѷ��еȣ�����֪ʶ��϶ࡢ�ۺ��Խ�ǿ����ֿ�����ѧ���ķ���������������ע�����ճ����л���ṹ�����ʣ���ȷ�л�������ԭ�ṹ��ʽ��ȷ��������

��ϰ��ϵ�д�
�����Ŀ

 ijͬѧͨ���������ϵ�֪�������������ڸ����·�Ӧ���õ���������Ӧ���������Ͻ�
ijͬѧͨ���������ϵ�֪�������������ڸ����·�Ӧ���õ���������Ӧ���������Ͻ�


