��Ŀ����
�л���G��һ�����ϣ���ϳ�·����ͼ��ʾ������D����Է�������Ϊ88���Ҹ÷����������ֲ�ͬ����ԭ�ӣ���ע��������A��B��C��D��E��F��G��Ϊ�л��ͬʱ������ijЩ������ʡ�ԣ�

��֪����CH3CH2Cl+NaOH��CH3CH2OH+NaCl
��R-CH=CH2
RCH2CH2OH
��1��д��A�Ľṹ��ʽ ��D�����������ŵ�����Ϊ ��
��2���ֱ�д���ڢں͢ݲ��ķ�Ӧ����ʽ ��
��3�����Ϸ�Ӧ����������ȡ����Ӧ���͵��� ��������ţ�
��4��D��ͬ���칹���к��С�-COO-���ṹ�Ĺ��� �֣�������D������

��֪����CH3CH2Cl+NaOH��CH3CH2OH+NaCl
��R-CH=CH2
| B2H6 |
| H2O2/OH |
��1��д��A�Ľṹ��ʽ
��2���ֱ�д���ڢں͢ݲ��ķ�Ӧ����ʽ
��3�����Ϸ�Ӧ����������ȡ����Ӧ���͵���
��4��D��ͬ���칹���к��С�-COO-���ṹ�Ĺ���
���㣺�л�����ƶ�
ר�⣺�л���Ļ�ѧ���ʼ��ƶ�
������B������������������D����DӦΪ�ᣬD����Է�������Ϊ88���Ҹ÷����������ֲ�ͬ����ԭ�ӣ���ṹ��ʽ ӦΪ��CH3��2CHCOOH����CΪ��CH3��2CHCHO��BΪ��CH3��2CHCH2OH���������Ϣ��֪AΪ��CH3��2C=CH2�����ݷ�Ӧ�ۡ��ܡ��ݵĹ�ϵ��֪��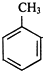 ��Cl2��Ӧ����EΪ
��Cl2��Ӧ����EΪ ��
�� ����ˮ������FΪ
����ˮ������FΪ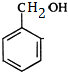 ��D��F����������Ӧ����GΪ
��D��F����������Ӧ����GΪ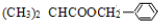 ���ݴ˴��⣻
���ݴ˴��⣻
 ��Cl2��Ӧ����EΪ
��Cl2��Ӧ����EΪ ��
�� ����ˮ������FΪ
����ˮ������FΪ ��D��F����������Ӧ����GΪ
��D��F����������Ӧ����GΪ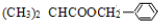 ���ݴ˴��⣻
���ݴ˴��⣻���
�⣺B������������������D����DӦΪ�ᣬD����Է�������Ϊ88���Ҹ÷����������ֲ�ͬ����ԭ�ӣ���ṹ��ʽ ӦΪ��CH3��2CHCOOH����CΪ��CH3��2CHCHO��BΪ��CH3��2CHCH2OH���������Ϣ��֪AΪ��CH3��2C=CH2�����ݷ�Ӧ�ۡ��ܡ��ݵĹ�ϵ��֪�� ��Cl2��Ӧ����EΪ
��Cl2��Ӧ����EΪ ��
�� ����ˮ������FΪ
����ˮ������FΪ ��D��F����������Ӧ����GΪ
��D��F����������Ӧ����GΪ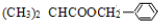 ��
��
��1����������ķ�����֪��A�Ľṹ��ʽΪ��CH3��2C=CH2��DΪ��CH3��2CHCOOH���������������ŵ�����Ϊ�Ȼ���
�ʴ�Ϊ����CH3��2C=CH2���Ȼ���
��2���������е�ת����ϵ��֪����Ӧ�ڵķ���ʽΪ��CH3��2CHCH2OH+O2
2��CH3��2CHCHO����Ӧ�ݵķ���ʽΪ��CH3��2CHCOOH+
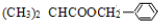 +H2O��
+H2O��
�ʴ�Ϊ����CH3��2CHCH2OH+O2
2��CH3��2CHCHO����CH3��2CHCOOH+
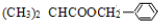 +H2O��
+H2O��
��3�������Ϸ�Ӧ�����У���Ӧ������±������ȡ����Ӧ����Ӧ��±��������ˮ����ȡ����Ӧ����Ӧ��������Ӧ��ȡ����Ӧ����Ϊ�ۢܢݣ�
�ʴ�Ϊ���ۢܢݣ�
��4����CH3��2CHCOOH��ͬ���칹���к��С�-COO-���ṹ���У�CH3CH2CH2COOH��CH3CH2COOCH3��CH3COOCH2CH3��HCOOCH2CH2CH3��HCOOCH��CH3��2����5�֣�
�ʴ�Ϊ��5��
 ��Cl2��Ӧ����EΪ
��Cl2��Ӧ����EΪ ��
�� ����ˮ������FΪ
����ˮ������FΪ ��D��F����������Ӧ����GΪ
��D��F����������Ӧ����GΪ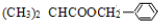 ��
����1����������ķ�����֪��A�Ľṹ��ʽΪ��CH3��2C=CH2��DΪ��CH3��2CHCOOH���������������ŵ�����Ϊ�Ȼ���
�ʴ�Ϊ����CH3��2C=CH2���Ȼ���
��2���������е�ת����ϵ��֪����Ӧ�ڵķ���ʽΪ��CH3��2CHCH2OH+O2
| Cu/Ag |
| �� |

| Ũ���� |
| �� |
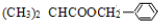 +H2O��
+H2O���ʴ�Ϊ����CH3��2CHCH2OH+O2
| Cu/Ag |
| �� |

| Ũ���� |
| �� |
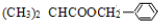 +H2O��
+H2O����3�������Ϸ�Ӧ�����У���Ӧ������±������ȡ����Ӧ����Ӧ��±��������ˮ����ȡ����Ӧ����Ӧ��������Ӧ��ȡ����Ӧ����Ϊ�ۢܢݣ�
�ʴ�Ϊ���ۢܢݣ�
��4����CH3��2CHCOOH��ͬ���칹���к��С�-COO-���ṹ���У�CH3CH2CH2COOH��CH3CH2COOCH3��CH3COOCH2CH3��HCOOCH2CH2CH3��HCOOCH��CH3��2����5�֣�
�ʴ�Ϊ��5��
���������⿼���л�����ƶϣ�ע���������л���Ľṹ����Է������������ƶϣ��������չ����ŵ�������ת���ǽ����Ĺؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��ǰ����ϵ�д�
��ǰ����ϵ�д�
�����Ŀ
��Ӧ2SO2+O2?2SO3��һ��ʱ���SO2��Ũ��������0.4mol/L�������ʱ������O2��ʾ�ķ�Ӧ����Ϊ0.04mol/��L?s���������ʱ��Ϊ��������
| A��0.1s | B��2.5s |
| C��5s | D��10s |
�������ʷ�Ӧ�����ӷ���ʽ����ȷ���ǣ�������
| A������ͭ��ϡ���ᷴӦ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O | ||||
| B����������ͭ��Һ��Ӧ��Cu2++2Na=2Na++Cu | ||||
| C����NaHCO3��Һ�м��������Ba��OH��2��Һ��HCO3-+Ba2++OH-=BaCO3��+H2O | ||||
D���ö��Ե缫��ⱥ���Ȼ�����Һ��2Cl-+2H2O
|
 ��ͼ��ʾ����ϡ������п��Ӧ�ⶨ��Ӧ���ʵ�װ�ã��ڷ�Һ©���м���ϡ���ᣬ����ƿ�м���п��ͨ���ⶨ����һ�����������õ�ʱ�����ⶨ��Ӧ�����ʣ�����50mL 1mol/L���ᣬ����ƿ�м������и���п����������ͬ��������H2�����ǣ�������
��ͼ��ʾ����ϡ������п��Ӧ�ⶨ��Ӧ���ʵ�װ�ã��ڷ�Һ©���м���ϡ���ᣬ����ƿ�м���п��ͨ���ⶨ����һ�����������õ�ʱ�����ⶨ��Ӧ�����ʣ�����50mL 1mol/L���ᣬ����ƿ�м������и���п����������ͬ��������H2�����ǣ�������| A������п�� |
| B������� |
| C��������ͭ���ʵ�п�� |
| D��������ͭ���ʵ�п�� |
 ij����С��������ͼ��ʾ��װ����ȡCl2���ṩ���Լ���Ũ���ᡢ����ʳ��ˮ��Cl2�������ܽ�Ƚ�С��������������Һ��������ع��壬��Ӧ�Ļ�ѧ����ʽΪ��2KMnO4+16HCl��Ũ��=2KCl+2MnCl2+5Cl2��+8H2O
ij����С��������ͼ��ʾ��װ����ȡCl2���ṩ���Լ���Ũ���ᡢ����ʳ��ˮ��Cl2�������ܽ�Ƚ�С��������������Һ��������ع��壬��Ӧ�Ļ�ѧ����ʽΪ��2KMnO4+16HCl��Ũ��=2KCl+2MnCl2+5Cl2��+8H2O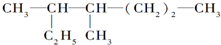 ��������
��������