��Ŀ����
7������ú̿���ϳɼ״��ķ�ӦΪCO��g��+2H2��g��?CH3OH��g�������ܱ������У���CO��H2�����ʵ���1��2��Ϸ�Ӧ��CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ���������߷ֱ��ʾѹǿΪ0.1MPa��5.0MPa��COת�������¶ȵı仯��
�ش��������⣺
A���÷�Ӧ���Է����е������ǵ��£�����¡����¡��κ��¶ȣ�
B��ͼ���������߷ֱ��ʾѹǿΪ0.1MPa��5.0MPa��COת�������¶ȵı仯�����д���ѹǿ��5.0MPa��������A���A����B������
C�����÷�Ӧ��һ�������´ﵽ��H=T��S�����ʱ��Ӧ��V��=V���������������|����=������
D.0.1MPa��200��ʱƽ���������м״������ʵ���������25%��
��ҵ�ϳɰ���Ӧ�У���ͨ����Ҫ�ļ���ͷ����ж����иı��������һ����ʹ�ϳɰ���ӦN2��g��+3H2��g��?2NH3��g���Ļ�ѧƽ���������ƶ�����ABD��
A�������¶Ⱥ�������䣬ͨ������N2
B�������¶Ⱥ�������䣬ͨ������H2
C�������¶Ⱥ�ѹǿ���䣬ͨ������N2
D�������¶Ⱥ�ѹǿ���䣬ͨ������H2
���п�����Ա���������ȼ�ϵ�أ���ع���ԭ����ͼ��ʾ��һ���缫ͨ����������һ�缫ͨ������������������Dz�����Y2O3��ZrO2���壬���ڸ������ܴ���O2-��
��1���Լ��飨C6H14���������ͣ�д���õ�ع���ʱ������Ӧ����ʽC6H14-38e-+19O2-=6CO2+7H2O��
��2����֪1mol���ӵĵ�����96500C���øõ�غͶ��Ե缫��ⱥ��ʳ��ˮ������·��ͨ��1.9300��104C�ĵ���ʱ�����ɱ�������������Ϊ22.4L��

���� ��A���Է����е��ж������ǡ�H-T��S��0����Ϸ�Ӧ���������ж���Ҫ��������
B���Ƚ�ѹǿ��ͳһ�¶ȣ�����ͬ�¶�200��ʱ��A��ת���ʱ�B�ĸߣ�˵��ѹǿ�ı䣬ƽ�������ƶ���
C����G=��H-T��S=0��˵���˷�Ӧ�ﵽƽ��״̬��
D�����ͼ����������ʽ�������⣻
��A�����ӷ�Ӧ���Ũ�ȣ���ѧ��Ӧ���ʼӿ죬��ѧƽ�������ƶ���
B��Ҳ�����ӷ�Ӧ���Ũ�ȣ���ѧƽ�������ƶ���
C��ͨ����Ҫ�ļ���ͷ����жϣ�����ij���£���ӦN2��g��+3H2��g��?2NH3��g����ij��ѹ�������з����ϳɰ��ķ�Ӧ����ƽ��ʱ��������к���1.0molN2��0.4molH2��0.4molNH3����ʱ�ݻ�Ϊ2���������¶Ⱥ�ѹǿ���䣬���֮�ȵ��������ʵ���֮�ȣ����������ͨ��0.36mol N2����ʽ����ƽ��Ũ�ȣ�
D��ͨ����Ҫ�ļ���ͷ����жϣ�����ij���£���ӦN2��g��+3H2��g��?2NH3��g����ij��ѹ�������з����ϳɰ��ķ�Ӧ����ƽ��ʱ��������к���1.0molN2��0.4molH2��0.4molNH3����ʱ�ݻ�Ϊ2���������¶Ⱥ�ѹǿ���䣬���֮�ȵ��������ʵ���֮�ȣ����������ͨ��0.36mol H2����ʽ����ƽ��Ũ�ȣ�
��1����������ڸ������ܴ���O2-����������������Ӧ����C6H14ʧȥ��������CO2�����������غ�͵���غ�д���缫��Ӧʽ��
��2��һ�����ӵĵ�����1.602��10-19C������·��ͨ��1.929��105 C�ĵ���ʱ�����ӵĸ���=$\frac{1.929��1{0}^{5}C}{1.602��1{0}^{-19}C}$=1.204��1024�����ӵ����ʵ���=$\frac{1.204��1{0}^{24}}{6.02��1{0}^{23}mo{l}^{-1}}$=2mol������ת�Ƶ��Ӻ������Ĺ�ϵʽ���㣮
��� �⣺��A����CO2��g��+3H2��g���TCH3OH��l��+H2O��l�����ر��S��0����Ӧ�ʱ��H��0�������������H-T��S��0��
�ʴ�Ϊ�����£�
B���Ƚ�ѹǿ��ͳһ�¶ȣ�����ͬ�¶�200��ʱ��A��ת���ʱ�B�ĸߣ�˵��ѹǿ�ı䣬ƽ�������ƶ�������CO��g��+2H2��g��?CH3OH��g���������������С�ķ�Ӧ����ѹǿ�ĸı�ֻ��������ѹǿ������A���ѹǿ��Ϊ5.0MPa��
�ʴ�Ϊ��A��
C����G=��H-T��S=0��˵����Ӧ�ﵽƽ��״̬�����ʱ��Ӧ��V��=V����
�ʴ�Ϊ��=��
D�������COamol������H22amol
����������������CO��g��+2H2��g��?CH3OH��g����
��Ӧǰ��mol�� ��a ������ 2a ����������0
��Ӧ�ˣ�mol�� 0.5a �� �� a �������� ��0.5a
ƽ��ʱ��mol�� 0.5a���� a �������� ��0.5a
����ã�$\frac{0.5a}{0.5a+a+0.5a}$��100%=25%��
�ʴ�Ϊ��25%��
��A�������¶Ⱥ�������䣬ͨ������N2�����ӷ�Ӧ�ﵪ����Ũ�ȣ���ѧ��Ӧ���ʼӿ죬��ѧƽ�������ƶ�����A��ȷ��
B�������¶Ⱥ�������䣬ͨ������H2��Ҳ�����ӷ�Ӧ���Ũ�ȣ���ѧƽ�������ƶ�����B��ȷ��
C������ij���£���ӦN2��g��+3H2��g��?2NH3��g����ij��ѹ�������з����ϳɰ��ķ�Ӧ����ƽ��ʱ��������к���1.0molN2��0.4molH2��0.4molNH3����ʱ�ݻ�Ϊ2������ʽ����ƽ��Ũ�ȣ�
N2��g��+3H2��g��?2NH3��g��
��ʼ����mol/L�� 0.6 0.5 0
�仯����mol/L�� 0.1 0.3 0.2
ƽ������mol/L�� 0.5 0.2 0.2
��������µ�ƽ�ⳣ��K=$\frac{0��{2}^{2}}{0.5��0��{2}^{3}}$=10��
�����¶Ⱥ�ѹǿ���䣬���֮�ȵ��������ʵ���֮�ȣ����������ͨ��0.36mol N2����ʱ�������Ϊ��1.0mol+0.4mol+0.4mol������1.0mol+0.4mol+0.4mol+0.36mol��=2��V��V=2.4L������Q��c��=$\frac{��\frac{0.4}{2.4}��^{2}}{\frac{1+0.36}{2.4}����\frac{0.4}{2.4}��^{3}}$=10.58��K=10��ƽ��������У���C����
D������ij���£���ӦN2��g��+3H2��g��?2NH3��g����ij��ѹ�������з����ϳɰ��ķ�Ӧ����ƽ��ʱ��������к���1.0molN2��0.4molH2��0.4molNH3����ʱ�ݻ�Ϊ2������ʽ����ƽ��Ũ�ȣ�
N2��g��+3H2��g��?2NH3��g��
��ʼ����mol/L�� 0.6 0.5 0
�仯����mol/L�� 0.1 0.3 0.2
ƽ������mol/L�� 0.5 0.2 0.2
��������µ�ƽ�ⳣ��K=$\frac{0��{2}^{2}}{0.5��0��{2}^{3}}$=10��
�����¶Ⱥ�ѹǿ���䣬���֮�ȵ��������ʵ���֮�ȣ����������ͨ��0.36mol H2����ʱ�������Ϊ��1.0mol+0.4mol+0.4mol������1.0mol+0.4mol+0.4mol+0.36mol��=2��V��V=2.4L������Q��c��=$\frac{��\frac{0.4}{2.4}��^{2}}{\frac{1}{2.4}����\frac{0.4+0.36}{2.4}��^{3}}$=2.1��K=10��ƽ��������У���D��ȷ��
��ѡABD��
��1����������ڸ������ܴ���O2-����������������Ӧ����1molC6H14ʧȥ��������CO2����ʧȥ38mole-���缫��ӦΪ��C6H14-38e-+19O2-=6CO2+7H2O��
�ʴ�Ϊ��C6H14-38e-+19O2-=6CO2+7H2O��
��2��һ�����ӵĵ�����1.602��10-19C������·��ͨ��1.929��105 C�ĵ���ʱ�����ӵĸ���=$\frac{1.929��1{0}^{5}C}{1.602��1{0}^{-19}C}$=1.204��1024�����ӵ����ʵ���=$\frac{1.204��1{0}^{24}}{6.02��1{0}^{23}mo{l}^{-1}}$=2mol������ת�Ƶ��Ӻ������Ĺ�ϵʽ�����������ʵ���Ϊ1mol�������Ϊ22.4L��
�ʴ�Ϊ��22.4��
���� ���⿼�鷴Ӧ�Է��Ե��жϡ���ѧƽ��״̬�жϣ��漰Ӱ�컯ѧƽ��ļ��㡢�Ȼ�ѧ����ʽ�Լ�����֪ʶ���ѶȽϴ���ƽ�⽨����;�����бȽ��ǹؼ�����Ͽ����˻�ѧ��Ӧ���ʺͶ�ͼ������������������ۺ�����Ҫ��ϸߣ�

| A�� | H2 | B�� | AlCl3 | C�� | CH4 | D�� | H2SO4 |
| �� | �� | �� | �� | ||
| �ܱ��������/L | 2 | 2 | 2 | 1 | |
| ��ʼ���ʵ��� | n��SO2��/mol | 0.4 | 0.8 | 0.8 | 0.4 |
| n��O2��/mol | 0.24 | 0.24 | 0.48 | 0.24 | |
| SO2��ƽ��ת����/% | 80 | ��1 | ��2 | ��3 | |
| A�� | SO2��ƽ��ת���ʣ���1����2=��3 | B�� | SO3�����ʵ���Ũ�ȣ�c���ף�=c��������c������ | ||
| C�� | �ס����е�ƽ�ⳣ����K���ף�=K���ң�=400 | D�� | �������е�ƽ�ⳣ����K��������K������ |
��1����֪��2N2H4��1��+N2O4��1���T3N2��g��+4H2O��1����H=-1225kJ•mol-1
�Ͽ�1mol���л�ѧ�����յ������ֱ�Ϊ��N-H��391kJ��N-N��193kJ��N��N��946kJ��O-H��463kJ��
��ʹ1molN2O4��1�������л�ѧ����ȫ����ʱ��Ҫ���յ�������1803KJ��
��2��t��ʱ����һ������NO2��g����N2O4��g������һ���ݻ�Ϊ2L�ĺ����ܱ������У�Ũ����ʱ��仯��ϵ�����ʾ��
| ʱ�� | 0 | 5 | 10 | 15 | 20 | 25 | 30 |
| c��X��/mol•L-1 | 0.2 | c | 0.6 | 0.6 | 1.0 | c1 | c1 |
| c��Y��/mol•L-1 | 0.6 | c | 0.4 | 0.4 | 0.4 | c2 | c2 |
��ǰ10min����NO2��ʾ�ķ�Ӧ����Ϊ0.04mol/��L•min����20minʱ�ı������������NO2��Ũ�ȣ����������м���0.8mol���������������´ﵽƽ��ʱ��NO2�İٷֺ�����ԭƽ��״̬���B������ţ�
A����������B����С��������C�����䡡����D�����ж�
��3���µ������백���ƣ���ˮ��Һ�������ԣ����õ��뷽��ʽ��ʾ�µ�ˮ��Һ�Լ��Ե�ԭ��N2H4+H2O?N2H+5+OH-�������������ɵ�ȼ�ϵ���ڼ��������·ŵ�ʱ������ˮ��һ������Ⱦ�����壮�ŵ�ʱ���õ�ظ����ĵ缫��ӦʽΪN2H4+4OH--4e-=4H2O+N2��
��4����֪����ͬ������N2H4•H2O�ĵ���̶ȴ���N2H5Cl��ˮ��̶ȣ������£�����0.2mo1•L-1N2H4•H2O��Һ��0.1mol•L-1HCl��Һ�������ϣ�����Һ��N2H5+��Cl-��OH-��H+����Ũ���ɴ�С��˳��Ϊc��N2H5+����c��Cl-����c��OH-����c��H+����
| A�� |  | B�� |  | C�� |  | D�� |  |
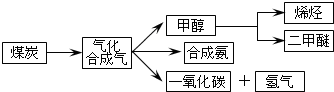
I����֪�ò�ҵ����ij��Ӧ��ƽ�����ʽΪ��K=$\frac{{c��{H_2}��•c��{CO}��}}{{c��{H_2}O��}}$��������Ӧ��Ӧ�Ļ�ѧ����ʽΪC��s��+H2O��g��?CO��g��+H2��g����
II�������ѣ�CH3OCH3����δ������������ͺ�Һ������Ϊ�ྻҺ��ȼ��ʹ�ã���ҵ����CO��H2Ϊԭ������CH3OCH3����ҵ�Ʊ��������ڴ���Ӧ���У�ѹ��2.0��10.0Mpa���¶�230��280�棩�������з�Ӧ��
��CO��g��+2H2��g��?CH3OH��g����H1=-90.7kJ•mol-1
��2CH3OH��g��?CH3OCH3��g��+H2O��g����H2=-23.5kJ•mol-1
��CO��g��+H2O��g��?CO2��g��+H2��g����H3=-41.2kJ•mol-1
��1������Ӧ�����ܷ�Ӧ���Ȼ�ѧ����ʽΪ3CO��g��+3H2��g��?CH3OCH3��g��+CO2��g����H=-247kJ•mol-1��830��ʱ��Ӧ�۵�K=1.0�����ڴ���Ӧ���з�Ӧ�۵�K��1.0�����������������=������
��2����ij�¶��£�����Ӧ�ٵ���ʼŨ�ȷֱ�Ϊ��c��CO��=1mol/L��c��H2��=2.4mol/L��5min��ﵽƽ�⣬CO��ת����Ϊ50%������Ӧ�����ʼŨ�ȷֱ�Ϊ��c��CO��=4mol/L��c��H2��=a mol/L���ﵽƽ���c��CH3OH��=2mol/L��a=5.4mol/L��
��3����Ӧ����t��ʱ��ƽ�ⳣ��Ϊ400�����¶��£���0.5L���ܱ������м���һ�����ļ״�����Ӧ��ijʱ�̲�ø���ֵ����ʵ���Ũ�����£�
| ���� | CH3OH | CH3OCH3 | H2O |
| c/��mol•L-1�� | 0.8 | 1.24 | 1.24 |
��ƽ��ʱ�����ѵ����ʵ���Ũ����1.6mol/L��
| A�� | �÷�Ӧ����������ԭ��Ӧ | B�� | ����ֻ���������� | ||
| C�� | N2O4�ǻ�ԭ�� | D�� | N2O4�������� |
