��Ŀ����
��ҵ����Ҫ����һ�����յĺ�ͭ�������쵨����CuSO4?5H2O�����÷����и��ֳɷݺ������£�Cu��CuOԼռ87%������ΪAl��Fe��Si��������������������������ʣ���������Ϊ��
���ֽ���������������������ʽ��ȫ����ʱ��Һ��pH���±���
�ش�
��1���������ΪʲôҪ����ͭ������ĥ�ɷ�״�� ��
��2���������H2O2�μӷ�Ӧ�����ӷ���ʽ�� ��
��3��������е�����ҺpHʱ���˵������� ��������ţ���
A��NaOH B����ˮ C��Cu2��OH��2CO3 D��Cu��OH��2 E��MgCO3
������������ɷݵĻ�ѧʽ ��
��4������������������pH=2��3��ԭ���� ��
��5�������ķ�������ǣ� ����ȴ�ᾧ�� ��
| �ٽ���ͭ�����гɷ�ĩ | - | �ڼӹ����ȼ���Һ������ | - | ��������ˮϴ��pH=7 | - | �ܼ��Թ���ϡ���ᣬ����������ͨ������������������ܽ� |
| �����õ��������� | �� | �����ٽ���Һ���������pH=2��3 | ����pH=5.2����1Сʱ֮����� | �� | ���ټ�H2O2��80�汣�°�Сʱ |
| ������ | Al��OH��3 | Fe��OH��3 | Cu��OH��2 | Mg��OH��2 | Fe��OH��2 |
| pH | 5.2 | 3.1 | 6.7 | 9.4 | 9.7 |
��1���������ΪʲôҪ����ͭ������ĥ�ɷ�״��
��2���������H2O2�μӷ�Ӧ�����ӷ���ʽ��
��3��������е�����ҺpHʱ���˵�������
A��NaOH B����ˮ C��Cu2��OH��2CO3 D��Cu��OH��2 E��MgCO3
������������ɷݵĻ�ѧʽ
��4������������������pH=2��3��ԭ����
��5�������ķ�������ǣ�
���㣺�����Ļ����뻷������Դ����,���ܵ���ʵ��ܽ�ƽ�⼰����ת���ı���,ͭ����������Ҫ���������Ҫ����
ר�⣺ʵ����
��������1����Ӧ��ĽӴ�����뷴Ӧ���ʳ����ȣ�
��2�������������ǿ�����ԣ����������������Ϊ�����ӣ�
��3������pH���������µ����ʣ����������ӳ�����pH��֪���ɵij�����
��4������ͭ���ӵ�ˮ�⣻
��5������Һ�еõ�����һ���ȡ����Ũ������ȴ�ᾧ�����˵ķ�����
��2�������������ǿ�����ԣ����������������Ϊ�����ӣ�
��3������pH���������µ����ʣ����������ӳ�����pH��֪���ɵij�����
��4������ͭ���ӵ�ˮ�⣻
��5������Һ�еõ�����һ���ȡ����Ũ������ȴ�ᾧ�����˵ķ�����
���
�⣺��1����Ӧ��ĽӴ�����뷴Ӧ���ʳ����ȣ�����ͭ������ĥ�ɷ�״��������巴Ӧ��ĽӴ�������ӿ��˷�Ӧ���ʣ�
�ʴ�Ϊ��������巴Ӧ��ı����������ѧ��Ӧ�����ʣ�
��2�������������ǿ�����ԣ����������������Ϊ�����ӣ���������ԭΪˮ�����ӷ���ʽΪ��2Fe2++H2O2+2H+=2Fe3++2H2O��
�ʴ�Ϊ��2Fe2++H2O2+2H+=2Fe3++2H2O��
��3������pH���������µ����ʣ�A������������ӣ�B�������笠����ӡ�E�������þ���ӣ�C��D�����ʻ�������ӷ�Ӧ����pH��ͬʱ����ͭ���ӣ����������µ����ʣ����������ӳ�����pH��֪����pHΪ5.2ʱ�������ӳ�����ȫ��������������
�ʴ�Ϊ��C��D��Fe��OH��3��
��4��ͭ����Ϊ������������ˮ�⣬ˮ�ⷽ��ʽΪ��Cu2++2H2O?Cu��OH��2+2H+������ʱ��ٽ�ˮ�⣬�������ᣬ������H+����Ũ�ȣ�������ͭ����ˮ�⣬
�ʴ�Ϊ����������Ũ��ʱ�ᵼ��Cu2+����ˮ������Cu��OH��2�����������pH=2��3��Ϊ������Cu2+��ˮ�⣬Ҳ�����������ʣ�
��5������Һ�еõ�����һ���ȡ����Ũ������ȴ�ᾧ�����˵ķ�����
�ʴ�Ϊ������Ũ�������ˣ�
�ʴ�Ϊ��������巴Ӧ��ı����������ѧ��Ӧ�����ʣ�
��2�������������ǿ�����ԣ����������������Ϊ�����ӣ���������ԭΪˮ�����ӷ���ʽΪ��2Fe2++H2O2+2H+=2Fe3++2H2O��
�ʴ�Ϊ��2Fe2++H2O2+2H+=2Fe3++2H2O��
��3������pH���������µ����ʣ�A������������ӣ�B�������笠����ӡ�E�������þ���ӣ�C��D�����ʻ�������ӷ�Ӧ����pH��ͬʱ����ͭ���ӣ����������µ����ʣ����������ӳ�����pH��֪����pHΪ5.2ʱ�������ӳ�����ȫ��������������
�ʴ�Ϊ��C��D��Fe��OH��3��
��4��ͭ����Ϊ������������ˮ�⣬ˮ�ⷽ��ʽΪ��Cu2++2H2O?Cu��OH��2+2H+������ʱ��ٽ�ˮ�⣬�������ᣬ������H+����Ũ�ȣ�������ͭ����ˮ�⣬
�ʴ�Ϊ����������Ũ��ʱ�ᵼ��Cu2+����ˮ������Cu��OH��2�����������pH=2��3��Ϊ������Cu2+��ˮ�⣬Ҳ�����������ʣ�
��5������Һ�еõ�����һ���ȡ����Ũ������ȴ�ᾧ�����˵ķ�����
�ʴ�Ϊ������Ũ�������ˣ�
����������������ͼ����ʽ���������ʵķ������ᴿ�����ӷ���ʽ����д��ˮ��ƽ���֪ʶ��ע����ȡ�������壬��Cu2��OH��2CO3��Cu��OH��2����pH����ȥ���ʣ�������ᴿʱһ�����������µ����ʣ�����pHһ����뺬�ᴿ�����е����ӵĻ�������������µ����ʣ���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
���и���������ָ��������һ���ܴ���������ǣ�������
| A���ں��д���I-���ӵ���Һ�У�Cl-��Fe3+��Na+��Mg2+ |
| B������ˮ�������c��H+��=10-12mol?L-1 ����Һ�У�Na+��Ba2+��Cl-��Br- |
| C��ʹ���ȳʺ�ɫ����Һ�У�Fe2+��Na+��SO42-��ClO- |
| D���ڼ���Al�ܷų�����H2����Һ�У�NH4+��SO42-��Cl-��NO3- |
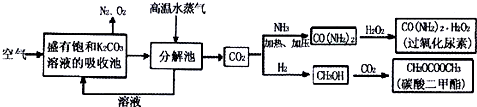
 ��ҵ�ϲ���SO2��N2��O2���������SO2������װ����ͼ����Ӧ����װ�е�ĵ�����Һ��SO2��I2�����ķ�ӦΪ��N2��O2����I2��Ӧ����SO2+I2+2H2O�TH2SO4+2HI
��ҵ�ϲ���SO2��N2��O2���������SO2������װ����ͼ����Ӧ����װ�е�ĵ�����Һ��SO2��I2�����ķ�ӦΪ��N2��O2����I2��Ӧ����SO2+I2+2H2O�TH2SO4+2HI ij��ѧ����С��Ӧ����ͼ��ʾ�ķ����о����ʵ����ʣ���������X����Ҫ�ɷ��������������ǿ�����ˮ�������ش��������⣮
ij��ѧ����С��Ӧ����ͼ��ʾ�ķ����о����ʵ����ʣ���������X����Ҫ�ɷ��������������ǿ�����ˮ�������ش��������⣮ ��1��������ȼ������ʱ������Ӧ��N2��g��+O2��g��?2NO��g�����ǵ�������β���к���NO��ԭ��֮һ��T��ʱ����5L�ܱ������г���6.5molN2��7.5molO2����5minʱ��Ӧ�ﵽƽ��״̬����ʱ������NO�����ʵ�����5mol��
��1��������ȼ������ʱ������Ӧ��N2��g��+O2��g��?2NO��g�����ǵ�������β���к���NO��ԭ��֮һ��T��ʱ����5L�ܱ������г���6.5molN2��7.5molO2����5minʱ��Ӧ�ﵽƽ��״̬����ʱ������NO�����ʵ�����5mol��