��Ŀ����
ʵ����������0.5mol/L��NaOH��Һ500ml������������
���ձ� ��100ml��Ͳ ��100ml����ƿ ��500ml����ƿ �ݲ����� ��������ƽ�������룩
��1������ʱ������ʹ�õ������� ������ţ�����ȱ�ٵ������� ���������������õ��������������÷ֱ��� �� ��
��2��ʹ������ƿǰ������е�һ�������� ��
��3��������Һʱ��һ����Է�Ϊ���¼������裺
�ٳ��� �ڼ��� ���ܽ� ��ҡ�� ��ת�� ��ϴ�� �߶��� ����ȴ
����ȷ�IJ���˳��Ϊ ��������ţ�
��4�������ƹ����У�������������ȷ�ģ����в������������ƫ�ߵ��� ��
��û��ϴ���ձ��Ͳ�����
��δ��NaOH��Һ��ȴ�����¾�ת�Ƶ�������
������ƿ�����������������ˮ
�ܶ���ʱ���ӱ��� �ݶ���ʱ���ӱ��ߣ�
���ձ� ��100ml��Ͳ ��100ml����ƿ ��500ml����ƿ �ݲ����� ��������ƽ�������룩
��1������ʱ������ʹ�õ�������
��2��ʹ������ƿǰ������е�һ��������
��3��������Һʱ��һ����Է�Ϊ���¼������裺
�ٳ��� �ڼ��� ���ܽ� ��ҡ�� ��ת�� ��ϴ�� �߶��� ����ȴ
����ȷ�IJ���˳��Ϊ
��4�������ƹ����У�������������ȷ�ģ����в������������ƫ�ߵ���
��û��ϴ���ձ��Ͳ�����
��δ��NaOH��Һ��ȴ�����¾�ת�Ƶ�������
������ƿ�����������������ˮ
�ܶ���ʱ���ӱ��� �ݶ���ʱ���ӱ��ߣ�
���㣺����һ�����ʵ���Ũ�ȵ���Һ
ר�⣺
��������1���������Ʋ����Ǽ��㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��������Ҫ��������ȱ�ٵ����������ݲ�������ʹ�����������ã�
��2������ƿ��ʹ��ǰ�����©��
��3���������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ���Բ���˳���������
��4������c=
��������ʵ����ʵ���n����Һ�����V�ı仯��������������
��2������ƿ��ʹ��ǰ�����©��
��3���������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ���Բ���˳���������
��4������c=
| n |
| V |
���
�⣺��1�����������м��㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣨������Ͳ��ȡˮ�����ձ��������ò��������裬�����ܽ⣮��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ���ձ���������2-3�Σ�����ϴ��Һ��������ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ���������������������ƽ���ձ�����������500mL����ƿ����ͷ�ιܣ�
�����ṩ��������֪������ʹ�õ������ǣ��٢ܢݢޣ���ȱ�ٵ������ǽ�ͷ�ιܣ�
������һ�����ʵ���Ũ����Һʱ�����ܽ�������Һʱ���õ������������÷ֱ��ǣ����裬�����ܽ��������
�ʴ�Ϊ���٢ܢݢޣ� ��ͷ�ιܣ� ���裬�����ܽ⣻ ������
��2������ƿ��ʹ��ǰ�����©���ʴ�Ϊ����©��
��3���������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��֪��ȷ�IJ���˳���ǣ��ڢ٢ۢ�ݢޢߢܣ��ʴ�Ϊ���ڢ٢ۢ�ݢޢߢܣ�
��4����û��ϴ���ձ��Ͳ��������ᵼ��������ʧ����Ũ��ƫ�ͣ��ʢٲ�ѡ��
��δ��NaOH��Һ��ȴ�����¾�ת�Ƶ������У�����ȴ����Һ���ƫС����Ũ��ƫ�ߣ��ʢ�ѡ��
��������ƿδ���T����������Һ������ҺŨ����Ӱ�죬��ΪֻҪ����ʱ��ȷ������ˮ��ԭ�����еĻ��Ǻ�������ģ���Ũ����Ӱ�죬�ʢ۲�ѡ��
�ܶ���ʱ���ӱ��ߣ��ᵼ����Һ���ƫС����Ũ��ƫ�ߣ��ʢ�ѡ��
�ݶ���ʱ���ӱ��ߣ��ᵼ����Һ���ƫ����Ũ��ƫ�ͣ��ʢݲ�ѡ��
��ѡ�ڢܣ�
�����ṩ��������֪������ʹ�õ������ǣ��٢ܢݢޣ���ȱ�ٵ������ǽ�ͷ�ιܣ�
������һ�����ʵ���Ũ����Һʱ�����ܽ�������Һʱ���õ������������÷ֱ��ǣ����裬�����ܽ��������
�ʴ�Ϊ���٢ܢݢޣ� ��ͷ�ιܣ� ���裬�����ܽ⣻ ������
��2������ƿ��ʹ��ǰ�����©���ʴ�Ϊ����©��
��3���������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��֪��ȷ�IJ���˳���ǣ��ڢ٢ۢ�ݢޢߢܣ��ʴ�Ϊ���ڢ٢ۢ�ݢޢߢܣ�
��4����û��ϴ���ձ��Ͳ��������ᵼ��������ʧ����Ũ��ƫ�ͣ��ʢٲ�ѡ��
��δ��NaOH��Һ��ȴ�����¾�ת�Ƶ������У�����ȴ����Һ���ƫС����Ũ��ƫ�ߣ��ʢ�ѡ��
��������ƿδ���T����������Һ������ҺŨ����Ӱ�죬��ΪֻҪ����ʱ��ȷ������ˮ��ԭ�����еĻ��Ǻ�������ģ���Ũ����Ӱ�죬�ʢ۲�ѡ��
�ܶ���ʱ���ӱ��ߣ��ᵼ����Һ���ƫС����Ũ��ƫ�ߣ��ʢ�ѡ��
�ݶ���ʱ���ӱ��ߣ��ᵼ����Һ���ƫ����Ũ��ƫ�ͣ��ʢݲ�ѡ��
��ѡ�ڢܣ�
���������⿼����һ�����ʵ���Ũ����Һ�����ƹ����еļ���������������ڻ�������Ŀ���ѶȲ���

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
�������ӷ���ʽ��ȷ���ǣ�������
| A�������ʯ��ˮ�м�������ʵ�����NaHCO3��Һ��Ca2++2OH-+2HCO3-=CaCO3��+CO32-+2H2O |
| B����NH4HSO4ϡ��Һ����μ���Ba��OH��2ϡ��Һ��SO42-�պó�����ȫ��Ba2++2OH-+NH4++H++SO42-=BaSO4��+NH3?H2O+H2O |
| C���������������������Fe��OH��3+3H+=Fe3++3H2O |
| D���Ȼ�����Һ�м��������ˮAl3++4OH-=AlO2-+2H2O |
��8�����ʣ��ټ��顢�ڱ����۾���ϩ����1��3-����ϩ����2-��Ȳ�����顢���ѽ�����������ʹ���Ը��������Һ��ɫ���ǣ�������
| A���٢ڢ� | B���ڢޢ� |
| C���ۢܢ� | D���ܢݢ� |
�����й����ʵ����ʵ�˵����ȷ���ǣ�������
| A�����ʯ��C60���нϴ��Ӳ�Ⱥͽϸߵ��۵� |
| B����ϡ�����м���ͭ�ۣ�ͭ�۲��ܽ⣻�ټ���Cu��NO3��2���壬ͭ���Բ��ܽ� |
| C��Cl2��SO2������ʹƷ����Һ��ɫ |
| D��þ�����Ƚ�����һ����������ˮ��Ӧ�����ɶ�Ӧ��������������� |
������Һ���й��������ʵ���Ũ�ȹ�ϵ��ȷ���ǣ�������
| A������������ʵ���Ũ�ȵ�NaClO��aq����NaCl��aq��������������Nǰ��N�� |
| B��NaHSO3��NaHCO3�����Ի����Һ�У�S��C����R��ʾ����c��Na+����c��HRO3-��+c��RO32-�� |
| C�������½������ơ���������Һ��Ϻ���Һ�����ԣ�������Һ�У�c��Na+����c��Cl-����c��CH3COOH�� |
| D�������£���0.1mol?L-1NH4Cl��Һ��0.05mol?L-1NaOH��Һ�������ϣ�c��C1-����c��Na+����c��NH4+����c��OH-����c��H+�� |
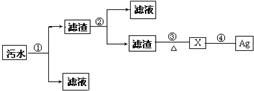
 ʵ������һ���������Ҵ��������Ũ����Ļ��Һ�Ʊ�����������װ����ͼ��ʾ��
ʵ������һ���������Ҵ��������Ũ����Ļ��Һ�Ʊ�����������װ����ͼ��ʾ��