��Ŀ����
3�����н���ʵ������ķ�Ӧ����ʽ��ȷ���ǣ�������| A�� | �п��Ľ���Na��¶�ڿ����У����������䰵2Na+O2�TNa2O | |
| B�� | ��AgCl����Һ�еμ�NaI��Һ����ɫ������ɻ�ɫAgCl+I-�TAgI+Cl- | |
| C�� | Na2O2�ڳ�ʪ�Ŀ����з���һ��ʱ�䣬��ɰ�ɫճ����2Na2O2+2CO2�T2Na2CO3+O2 | |
| D�� | ��NaHCO3��Һ�м�������ij���ʯ��ˮ�����ְ�ɫ����2HCO3-+Ca2++2OH-�TCaCO3��+CO32-+2H2O |
���� A��ԭ�Ӹ������غ㣻
B���⻯���ܽ��������Ȼ�������AgCl����Һ�еμ�NaI��Һ��ʵ���Ȼ���ת��Ϊ�⻯����ת����
C��Na2O2�ڳ�ʪ�Ŀ����з���һ��ʱ�䣬��ɰ�ɫճ��������Ϊ����������ˮ��Ӧ�����������ƣ��������Ƴ�������
D���������ƹ�����Ӧ����̼��ơ��������ƺ�ˮ��
��� �⣺A���п��Ľ���Na��¶�ڿ����У����������䰵����ѧ����ʽ��4Na+O2�TN2a2O����A����
B����AgCl����Һ�еμ�NaI��Һ����ɫ������ɻ�ɫ�����ӷ���ʽ��AgCl+I-�TAgI+Cl-����B��ȷ��
C��Na2O2�ڳ�ʪ�Ŀ����з���һ��ʱ�䣬��ɰ�ɫճ�����ѧ����ʽ��2Na2O2+2H2O�T4NaOH+O2������C����
D����NaHCO3��Һ�м�������ij���ʯ��ˮ�����ְ�ɫ���������ӷ���ʽ��HCO3-+Ca2++OH-�TCaCO3��+H2O����D����
��ѡ��B��
���� ���⿼���˷���ʽ�����ӷ���ʽ����д����ȷ���ʵ����ʼ�������Ӧ��ʵ���ǽ���ؼ���ע�ⷽ��ʽ�����ӷ���ʽ��д��ѭ�Ĺ��ɺͷ�������Ŀ�ѶȲ���

��ϰ��ϵ�д�
 �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д�
�����Ŀ
2�������й�ʵ�����������ͽ��ͻ���۶���ȷ���ǣ�������
| ѡ�� | ʵ����� | ���� | ���ͻ���� |
| A | ������AgNO3��Һ���ȵμ�����NaCl��Һ����μ�����KI��Һ | �Ȳ�����ɫ�������ֲ�����ɫ���� | AgI��AgCl������ |
| B | ��������ռ���Ҵ���Һ���� | �����������ʹ���Ը��������Һ��ɫ | ֤������ϩ���� |
| C | ������ˮ����ͨ�����ȵ����� | ���۱�� | ����ˮ�ڸ������ܷ�����Ӧ |
| D | Fe���м��������ϡHNO3����ַ�Ӧ����뼸��KSCN��Һ | ��Һ�ʺ�ɫ | ϡHNO3��Fe����ΪFe3+ |
| A�� | A | B�� | B | C�� | C | D�� | D |
11��NAΪ�����ӵ����������ж�0.3mol/L��K2SO4��Һ��˵���У���ȷ���ǣ�������
| A�� | 1L��Һ�к�0.3NA�������� | B�� | 1L��Һ�к�0.9NA�����ӣ�K+��SO42-�� | ||
| C�� | 2L��Һ�м�����Ũ����1.2mol/L | D�� | 2L��Һ�к�0.6 NA�����ӣ�K+��SO42-�� |
18������ʵ�鷽����ʵ�������ȷ���ǣ�������
| A�� |  ��ȥS02�е�����HC1���ɽ��������ͨ�˱���Na2S03��Һ ��ȥS02�е�����HC1���ɽ��������ͨ�˱���Na2S03��Һ | |
| B�� |  ճ���Թ��ڱڵ����ʣ������ȵ�ϡ����ϴ�� ճ���Թ��ڱڵ����ʣ������ȵ�ϡ����ϴ�� | |
| C�� |  ϡ���ᡢNaOH��AICl3��Ba��OH��2��ƿ��ɫ��Һ������NaHC03��Һ���� ϡ���ᡢNaOH��AICl3��Ba��OH��2��ƿ��ɫ��Һ������NaHC03��Һ���� | |
| D�� |  ������茶�������ˮ�����ˮ���½���֤�������ˮ�������ȵ� ������茶�������ˮ�����ˮ���½���֤�������ˮ�������ȵ� |
8���������ƣ�NaNO2����һ�ֹ�ҵ�Σ�ʵ���ҿ�������װ�ã���ȥ���ּг��������Ʊ���
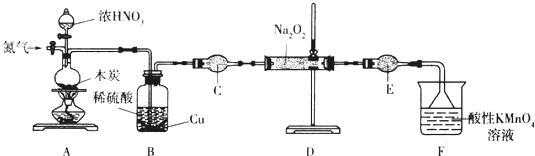
��֪����2NO+Na2O2�T2NaNO2��
��3NaNO2+3HCl�T3NaCl+HNO3+2NO��+H2O��
�����������£�NO��NO2������MnO4-��Ӧ����NO3-��Mn2+��Na2O2��ʹ���Ը��������Һ��ɫ��
��1������װ��Aǰ����ͨһ��ʱ��N2��Ŀ�����ų�װ���еĿ�����
��2��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪC+4HNO3��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CO2��+4NO2��+2H2O��ʵ�������Bƿ�е���Һ������Ũ������ȴ�ᾧ����������ƣ������˿ɻ��CuSO4•5H2O��
��3������C������Ϊ����ܣ�����ʢ�ŵ�ҩƷΪ��ʯ�ң������ƣ���
��4����ַ�Ӧ����װ��D�в���ķ����ǣ�ȡ�������������Թ��У�����ϡ������Һ�������ݲ��������Թܿ��Ϸ����ֺ���ɫ���壬�������NaNO2��ע���Լ�������
��5��Ϊ�ⶨ�������Ƶĺ�������ȡ4.000g��Ʒ����ˮ���250mL��Һ��ȡ25.00mL��Һ����ƿ�У���0.1000mol•L-1����KMnO4��Һ���еζ���ʵ�������������±���ʾ��
�ٵ�һ��ʵ�����ݳ����쳣����������쳣��ԭ�������ac������ţ���
a����ʽ�ζ���������ˮϴ����δ�ñ�Һ��ϴ
b����ƿϴ����δ����
c���ζ��յ�ʱ���Ӷ���
�ڸ��ݱ������ݣ��������ù������������Ƶ���������86.25%��
��6����ƺ���ʵ��Ƚ�0.1mol•L-1NaNO2��Һ��NO2-��ˮ��̶Ⱥ�0.1mol•L-1HNO2��Һ��HNO2�ĵ���̶���Դ�С������Ҫ˵��ʵ�鲽�衢����ͽ��ۣ�������ҩƷ��ѡ��25��C��0.1mol/LHNO2��0.1mol/LNaNO2��Һ�������ϣ����ⶨ��ҺPH��7��˵��HNO2�ĵ���̶ȴ���NO2-���ӵ�ˮ��̶ȣ����ⶨ��ҺpH��7��˵��NO2-����ˮ��̶ȴ���HNO2�ĵ���̶ȣ�

��֪����2NO+Na2O2�T2NaNO2��
��3NaNO2+3HCl�T3NaCl+HNO3+2NO��+H2O��
�����������£�NO��NO2������MnO4-��Ӧ����NO3-��Mn2+��Na2O2��ʹ���Ը��������Һ��ɫ��
��1������װ��Aǰ����ͨһ��ʱ��N2��Ŀ�����ų�װ���еĿ�����
��2��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪC+4HNO3��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CO2��+4NO2��+2H2O��ʵ�������Bƿ�е���Һ������Ũ������ȴ�ᾧ����������ƣ������˿ɻ��CuSO4•5H2O��
��3������C������Ϊ����ܣ�����ʢ�ŵ�ҩƷΪ��ʯ�ң������ƣ���
��4����ַ�Ӧ����װ��D�в���ķ����ǣ�ȡ�������������Թ��У�����ϡ������Һ�������ݲ��������Թܿ��Ϸ����ֺ���ɫ���壬�������NaNO2��ע���Լ�������
��5��Ϊ�ⶨ�������Ƶĺ�������ȡ4.000g��Ʒ����ˮ���250mL��Һ��ȡ25.00mL��Һ����ƿ�У���0.1000mol•L-1����KMnO4��Һ���еζ���ʵ�������������±���ʾ��
| ����� | 1 | 2 | 3 | 4 |
| KmnO4��Һ���/mL | 20.60 | 20.02 | 20.00 | 19.98 |
a����ʽ�ζ���������ˮϴ����δ�ñ�Һ��ϴ
b����ƿϴ����δ����
c���ζ��յ�ʱ���Ӷ���
�ڸ��ݱ������ݣ��������ù������������Ƶ���������86.25%��
��6����ƺ���ʵ��Ƚ�0.1mol•L-1NaNO2��Һ��NO2-��ˮ��̶Ⱥ�0.1mol•L-1HNO2��Һ��HNO2�ĵ���̶���Դ�С������Ҫ˵��ʵ�鲽�衢����ͽ��ۣ�������ҩƷ��ѡ��25��C��0.1mol/LHNO2��0.1mol/LNaNO2��Һ�������ϣ����ⶨ��ҺPH��7��˵��HNO2�ĵ���̶ȴ���NO2-���ӵ�ˮ��̶ȣ����ⶨ��ҺpH��7��˵��NO2-����ˮ��̶ȴ���HNO2�ĵ���̶ȣ�
15��ij��ѧ�о���ѧϰС����ⶨʳ����������g/10mL������������С���ʵ�鲢�ش�������⣮
[ʵ��Ŀ��]�ⶨʳ��������
[ʵ��ԭ��]�к͵ζ�
[ʵ����Ʒ]����ˮ������ʳ�ð״���Ʒ500mL���̱�ע����������3��.50g/100mL��5.00g/100mL����0.1000mol/L NaOH����Һ��100mL����ƿ��10mL��Һ�ܣ���ʽ�ζ��ܣ�����̨���ζ��ܼУ���ƿ���ձ������ָʾ�����ɹ�ѡ�õ��У����ȣ���̪��ʯ���
[ʵ�鲽��]
�����Ʋ���ȡ����ʳ����Һ
��10mL��Һ����ȡ10mL���۰״���Ʒ����100mL����ƿ�У��ô�����������ˮϡ�����̶��ߣ�ҡ�Ⱥ�����ʽ�ζ���ȡ����ʳ����Һ20mL����������ƿ�У�
��ʢװ��NaOH��Һ
����ʽ�ζ���ϴ������NaOH����Һ��ϴ3�Σ�Ȼ�����NaOH����Һ���ų����첿�����ݺ�ʹҺ��λ�ڡ�0���̶Ȼ�0�������£����ã���ȡ���ݲ���¼ΪNaOH����Һ����ij�������
�۵ζ�
��ʢ�д���ʳ����Һ����ƿ�еĵμ�ij���ָʾ��2��3�Σ��ζ����յ㣮��¼NaOH���ն������ظ��ζ�3�Σ�
[���ݼ�¼]
[������˼��]
��1�������������в�Ѹ����ȴ�ķ�����������ˮ��Ŀ���dz�ȥCO2����ֹ���ʵ������Ӱ�죮
��2�����������ѡ������ָʾ���Ƿ�̪��������CH3COONa��Һ�ʼ��ԣ�Ӧѡ���ɫ��Χ�ڼ��Ե�ָʾ����
��3����Ʒ������=4.50g/100mL��
��4������ʵ����ƫ�����Ҫԭ���Ǣ٢ڣ���д��ţ�
�ٵζ��յ�ʱ�����ӵζ��ܶ���
�ڵζ���ζ��ܼ��촦����һ��Һ��
����Һ��������ˮϴ����������ȡ����Һ
�ܵζ�ǰ����ʽ�ζ��������ݣ��ζ����������
����ƿֻ������ˮϴ�Ӻ���������������ˮ��
[ʵ��Ŀ��]�ⶨʳ��������
[ʵ��ԭ��]�к͵ζ�
[ʵ����Ʒ]����ˮ������ʳ�ð״���Ʒ500mL���̱�ע����������3��.50g/100mL��5.00g/100mL����0.1000mol/L NaOH����Һ��100mL����ƿ��10mL��Һ�ܣ���ʽ�ζ��ܣ�����̨���ζ��ܼУ���ƿ���ձ������ָʾ�����ɹ�ѡ�õ��У����ȣ���̪��ʯ���
[ʵ�鲽��]
�����Ʋ���ȡ����ʳ����Һ
��10mL��Һ����ȡ10mL���۰״���Ʒ����100mL����ƿ�У��ô�����������ˮϡ�����̶��ߣ�ҡ�Ⱥ�����ʽ�ζ���ȡ����ʳ����Һ20mL����������ƿ�У�
��ʢװ��NaOH��Һ
����ʽ�ζ���ϴ������NaOH����Һ��ϴ3�Σ�Ȼ�����NaOH����Һ���ų����첿�����ݺ�ʹҺ��λ�ڡ�0���̶Ȼ�0�������£����ã���ȡ���ݲ���¼ΪNaOH����Һ����ij�������
�۵ζ�
��ʢ�д���ʳ����Һ����ƿ�еĵμ�ij���ָʾ��2��3�Σ��ζ����յ㣮��¼NaOH���ն������ظ��ζ�3�Σ�
[���ݼ�¼]
| �ζ����� ʵ������ | 1 | 2 | 3 | 4 |
| V����Ʒ��/ml | 20.00 | 20.00 | 20.00 | 20.00 |
| V��NaOH��ʼ/ml | 0.00 | 0.20 | 0.10 | 0.10 |
| V��NaOH����/ml | 14.98 | 15.20 | 15.12 | 16.24 |
��1�������������в�Ѹ����ȴ�ķ�����������ˮ��Ŀ���dz�ȥCO2����ֹ���ʵ������Ӱ�죮
��2�����������ѡ������ָʾ���Ƿ�̪��������CH3COONa��Һ�ʼ��ԣ�Ӧѡ���ɫ��Χ�ڼ��Ե�ָʾ����
��3����Ʒ������=4.50g/100mL��
��4������ʵ����ƫ�����Ҫԭ���Ǣ٢ڣ���д��ţ�
�ٵζ��յ�ʱ�����ӵζ��ܶ���
�ڵζ���ζ��ܼ��촦����һ��Һ��
����Һ��������ˮϴ����������ȡ����Һ
�ܵζ�ǰ����ʽ�ζ��������ݣ��ζ����������
����ƿֻ������ˮϴ�Ӻ���������������ˮ��
13����ҵ����ȡ���ɰ�Ļ�ѧ����ʽ���£�SiO2+3C=SiC+2CO���������������ԭ��Ӧ�У��������ͻ�ԭ�������ʵ���֮��Ϊ��������
| A�� | 1��2 | B�� | 2��1 | C�� | 5��3 | D�� | 3��5 |
 ij��ѧ��ȤС��Ϊ̽��Ԫ�����ʵĵݱ���ɣ���ƿ�����ϵ��ʵ�飮
ij��ѧ��ȤС��Ϊ̽��Ԫ�����ʵĵݱ���ɣ���ƿ�����ϵ��ʵ�飮