��Ŀ����
14��������;�㷺�Ľ��������������������ײ����к��ĺ�����ҵ��ˮ��I����ԭ�������Ǵ�����Cr2O42- ��CrO72-��ҵ��ˮ��һ�ֳ��÷������乤���������£�
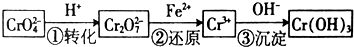
���еڢٲ�����ƽ�⣺2CrO42-����ɫ��+2H+?Cr2O72- ����ɫ��+H2O
��1����ƽ����ϵ�У�pH=0ʱ��Һ�Գ�ɫ��
��2����ʯīΪ�缫�����Na2CrO4�Ʊ�Na2Cr2O7��װ����ͼ1��ʾ��
a���������Ǹ�����b��ֱ������һ���ķ�ӦʽΪ2H2O-4e-=4H++O2����
��3���ڢڲ���Ӧ�����ӷ���ʽ��Cr2O72-+6Fe2++14H+=2Cr3++6Fe3++7H2O��
�ڢڲ���Ӧ�����Һ����һ�����ռ����Һ��c��Fe3+��=2.0��10-12mol•L-1������Һ��c��Cr3+��=3��10-5mol•L-1������֪Ksp[Fe��OH��3]=4.0��10-38 mol•L-1��Ksp[Cr��OH��3]=6.0��10-31 mol•L-1����
II��̽��CrO3��ǿ�����Ժ����ȶ���
��4��CrO3���л����ƾ���ʱ���ҷ�Ӧ�����Ż����ù������Ҵ������������ᣬCrO3����ԭ����ɫ��Cr2��SO4��3�����䲢��ƽ���з�Ӧʽ��
aCrO3+bC2H5OH+cH2SO4=dCr2��SO4��3+eCH3COOH+fR
b��c��f=1��2��3��
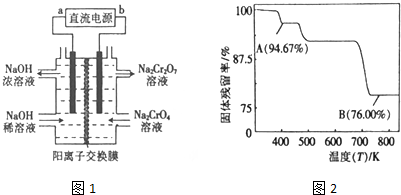
��5��CrO3�����ȶ��Բ����ʱ�ֽ⣬��̶����������¶ȵı仯��ͼ2��ʾ��B��ʱ���ɹ���Ļ�ѧʽΪCr2O3�������������=$\frac{ʣ����������}{ԭʼ���������}$��100%��
���� ��1�����������Ӷ�2CrO42-����ɫ��+2H+�TCr2O72- ����ɫ��+H2Oƽ���ƶ���Ӱ���жϣ�
��2������2CrO42-+2H+?Cr2O72-+H2O���ͼʾװ�ã���Ϊ���Ե缫�����Na2CrO4��Һ��ȡNa2Cr2O7����Ҫͨ���������H+�����Һ�����ԣ�˵���ڸõ缫�����������ӵ�Դ����������bΪ����������������������ʧ���ӣ�
��3������������Cr2O72-����������ԭ��Ӧ����c��Fe3+����Ksp[Fe��OH��3]����c��OH-�������Ksp[Cr��OH��3]����c��Cr3+����
��4�����ݻ��ϼ�������������Լ�ԭ���غ������
��5�����������غ㶨�ɣ��ڱ仯�����У�Cr������û�б䣬�����ԭ�Ӻ�ԭ�ӵĸ����ȼ��ɣ�
��� �⣺��1����ƽ����ϵ��pH=0����Һ���Խ�ǿ��ƽ��2CrO42-����ɫ��+2H+�TCr2O72- ����ɫ��+H2O�����ƶ�����Һ�ʳ�ɫ��
�ʴ�Ϊ���ȣ�
��2������2CrO42-+2H+?Cr2O72-+H2O���ͼʾװ�ã���Ϊ���Ե缫�����Na2CrO4��Һ��ȡNa2Cr2O7����Ҫͨ���������H+�����Һ�����ԣ�˵���ڸõ缫�����������ӵ�Դ����������bΪ��������aΪ����������������������ʧ���ӣ��������缫��ӦʽΪ4OH--4e-=O2��+2H2O��
�ʴ�Ϊ��������4OH--4e-=O2��+2H2O��
��3������������Cr2O72-����������ԭ��Ӧ����Ӧ�����ӷ���ʽΪ6Fe2++Cr2O72-+14H+=6Fe3++2Cr3++7H2O��
���Ӧ�����Һ��һ����NaOH������Һ��c��Fe3+��=2.0��10-12mol•L-1����c��OH-��=$\root{3}{\frac{4.0��1{0}^{-38}}{2.0��1{0}^{-12}}}$mol/L����c��Cr3+��=$\frac{6.0��1{0}^{-31}}{2.0��1{0}^{-26}}$=3��10-5��
�ʴ�Ϊ��6Fe2++Cr2O72-+14H+=6Fe3++2Cr3++7H2O��3��10-5��
��4��CrO3����ǿ�����ԣ������л����ƾ���ʱ���Ҵ������������ᣬ̼��ƽ�����ϼ۴�-2�����ߵ�0��1���Ҵ����ϼ۱仯4��CrO3����ԭ����ɫ�������[Cr2��SO4��3]�����Ļ��ϼ۴�+6�۽��͵�+3�ۣ�1��CrO3���ϼ۱仯3�����ߵ���С��������12���ٸ���ԭ���غ��4CrO3+3C2H5OH+6H2SO4=2Cr2��SO4��3+3CH3COOH+9H2O����b��c��f=1��2��3��
�ʴ�Ϊ��1��2��3��
��5�����������Ϊ100g��B��ʱ���������Ϊ��100g��76%=76g��Cr������û�б䣬������������Cr������Ϊ52g����Ԫ�ص�����Ϊ16�����ߵĸ�����Ϊ$\frac{52}{52}$��$\frac{24}{16}$=2��3������B��ʱʣ�����ijɷ���Cr2O3�����Լ��ȵ� 750K ʱ�ɷ���Cr2O3��
�ʴ�Ϊ��Cr2O3��
���� �����ۺϿ����˻�ѧƽ��ԭ����������ԭ��Ӧ�����ӷ���ʽ����д�������ܽ�ƽ���Ksp���йؼ�������ݣ������ڷ�Ӧԭ����Ӧ�õĿ��飬����ʱҪ���������Ϣ��������ԭ�����н����Ŀ�Ѷ��еȣ�

 ��ʵ�����У�������̼�����ơ��Ȼ��ơ��Ȼ�淋������ܽ�ȵIJ��죬ͨ������ʳ��ˮ�����Ͷ�����̼��Ӧ�����̼�����ƾ��壬��Ӧԭ���������»�ѧ����ʽ��ʾ��NH3+CO2+NaCl+H2O�TNH4Cl+NaHCO3�������ݴ�ԭ�������Ƶ�̼���ƾ��壬ijУѧ���������ͼʵ��װ�ã�����Bװ���е��Թ��������а����Ȼ��Ƶ���Һ���Ҷ��߾��Ѵﵽ���ͣ�
��ʵ�����У�������̼�����ơ��Ȼ��ơ��Ȼ�淋������ܽ�ȵIJ��죬ͨ������ʳ��ˮ�����Ͷ�����̼��Ӧ�����̼�����ƾ��壬��Ӧԭ���������»�ѧ����ʽ��ʾ��NH3+CO2+NaCl+H2O�TNH4Cl+NaHCO3�������ݴ�ԭ�������Ƶ�̼���ƾ��壬ijУѧ���������ͼʵ��װ�ã�����Bװ���е��Թ��������а����Ȼ��Ƶ���Һ���Ҷ��߾��Ѵﵽ���ͣ���1��Aװ������������Ӧ�����ӷ���ʽΪ��CaCO3+2H+=Ca2++CO2��+H2O��
Cװ����ϡ���������Ϊ�����մ�Bװ���е��Թ����ݳ��İ��������ٶԻ�������Ⱦ��
��2���������г�������������ڲ�ͬ�¶��µ��ܽ�����ݣ�g/100gˮ��
| 0�� | 10�� | 20�� | 30�� | 40�� | 50�� | |
| NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 |
| NaHCO3 | 6.9 | 8.1 | 9.6 | 11.1 | 12.7 | 14.5 |
| NH4Cl | 29.4 | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 |
��3����Уѧ���ڼ�������װ�������Ժ����ʵ�飬���û�еõ�̼�����ƾ��壬ָ����ʦָ��Ӧ��A��Bװ��֮�䣨��д��ĸ������һ��ʢ�б���NaHCO3��Һ ��ϴ��װ�ã��������dz�ȥCO2�л��е�HCl���壮
��4�����øĽ����װ�ý���ʵ�飬��B�е��Թ��������˾��壬����Ҫ�IJ�����õ���һ�ִ����ľ��壮��ͨ����ʵ���жϸþ�����̼�����ƾ��徧�������̼����茶��壬��������������ʵ�������ۣ�ȡ�������������Թ��У��ھƾ����ϼ���ʹ���ַ�Ӧ���а�ɫ����ʣ�࣬���岻��NH4HCO3��
��5������Уѧ������ʵ��ʱ�����ñ���ʳ��ˮ�к�NaCl������Ϊ5.85g��ʵ���õ������NaHCO3���������Ϊ5.88g����NaHCO3�IJ���Ϊ70%��
��1��������⣺Fe3+��Br2�ĸ������Ը�ǿ��
��2�����룺�ټ�ͬѧ��Ϊ�����ԣ�Fe3+��Br2��������ʵ�������Ƿ���������ԭ��Ӧ���£�����Һ�ʻ�ɫ�Ǻ�Br2���ѧʽ����ͬ�����£�
����ͬѧ��Ϊ�����ԣ�Br2��Fe3+�������������Ƿ���������ԭ��Ӧ���£�����Һ�ʻ�ɫ�Ǻ�Fe3+���£�
��3�����ʵ�鲢��֤��ͬѧΪ��֤��ͬѧ�Ĺ۵㣬ѡ������ijЩ�Լ���Ƴ����ַ�������ʵ�飬��ͨ���۲�ʵ������֤������ͬѧ�Ĺ۵�����ȷ�ģ�
��ѡ�õ��Լ���a����̪��Һ b��CCl4 c����ˮ�ƾ� d��KSCN��Һ��
�����ڱ���д����ͬѧѡ�õ��Լ���ʵ���й۲쵽������
| ѡ���Լ�������ţ� | ʵ������ | |
| ����1 | ||
| ����2 |
�����ԣ�Br2��Fe3+������������ϡ�Ȼ�������Һ�У�����1��2����ˮ����Һ�ʻ�ɫ�����������ӷ�Ӧ����ʽΪ2Fe2++Br2=2Fe3++2Br-��
��5��ʵ����˼��
�ٸ�������ʵ���Ʋ⣬�����廯������Һ��ͨ�����������ȱ�������������Fe2+��
����100mLFeBr2��Һ��ͨ��2.24LCl2����״��������Һ����$\frac{1}{2}$��Br-�������ɵ���Br2����ԭFeBr2��Һ�����ʵ���Ũ��Ϊ1mol/L��



 �����ȣ�SO2Cl2����һ����Ҫ���л��ϳ��Լ���ʵ���ҿ�����SO2��Cl2��Ӧ��ȡ������SO2Cl2��װ����ͼ����Щ�г�װ��ʡ�ԣ���ʾ����֪SO2Cl2���۵�Ϊ-54.1�棬�е�Ϊ69.1�棬��ˮ�ܷ������ҵ�ˮ�ⷴӦ��������������
�����ȣ�SO2Cl2����һ����Ҫ���л��ϳ��Լ���ʵ���ҿ�����SO2��Cl2��Ӧ��ȡ������SO2Cl2��װ����ͼ����Щ�г�װ��ʡ�ԣ���ʾ����֪SO2Cl2���۵�Ϊ-54.1�棬�е�Ϊ69.1�棬��ˮ�ܷ������ҵ�ˮ�ⷴӦ��������������