��Ŀ����
��֪�����ڵ�����Ԫ��X��Y��Z��W��M��ԭ��������������X�dz������ʵ���ҪԪ�أ�Yԭ��������ֻ��2�����ӣ�Z���ʿ��Ƴɰ뵼����ϣ�WԪ���γɵĵ���Ϊ��ɫ�Ĺ��壮��ش��������⣺
��1��Ԫ��W��̬ԭ�ӵĺ�������Ų�ʽΪ ��
��2��X��Y�γɵĻ������к��еĻ�ѧ��Ϊ ��
��3��Z�������ᄃ������Ϊ �� Z���������к��еĹ��ۼ�Ϊ �����Լ����Ǽ��Լ�����
��4��X��W�γɵ��⻯��ֱ�Ϊ���ң��Ҽס��������ĵ�������ȣ���ĽṹʽΪ ��
��5��W��M�ĵ����ܽϴ��Ϊ ����Ԫ�ط��ű�ʾ����
��1��Ԫ��W��̬ԭ�ӵĺ�������Ų�ʽΪ
��2��X��Y�γɵĻ������к��еĻ�ѧ��Ϊ
��3��Z�������ᄃ������Ϊ
��4��X��W�γɵ��⻯��ֱ�Ϊ���ң��Ҽס��������ĵ�������ȣ���ĽṹʽΪ
��5��W��M�ĵ����ܽϴ��Ϊ
���㣺λ�ýṹ���ʵ����ϵӦ��
ר�⣺Ԫ����������Ԫ�����ڱ�ר��
�����������ڵ�����Ԫ��X��Y��Z��W��M��ԭ��������������X�dz������ʵ���ҪԪ�أ���X��NԪ�أ�
Yԭ�ӵ������ֻ��2��������ԭ����������X������Y��MgԪ�أ�
Z���ʿ��Ƴɰ뵼�������Z��SiԪ�أ�WԪ���γɵĵ���Ϊ��ɫ�Ĺ��壬��W��SԪ�أ�
M�Ƕ���������Ԫ�أ���ԭ����������W������M��ClԪ�أ����Ԫ�ء����ʵĽṹ�����ʷ������
Yԭ�ӵ������ֻ��2��������ԭ����������X������Y��MgԪ�أ�
Z���ʿ��Ƴɰ뵼�������Z��SiԪ�أ�WԪ���γɵĵ���Ϊ��ɫ�Ĺ��壬��W��SԪ�أ�
M�Ƕ���������Ԫ�أ���ԭ����������W������M��ClԪ�أ����Ԫ�ء����ʵĽṹ�����ʷ������
���
�⣺�����ڵ�����Ԫ��X��Y��Z��W��M��ԭ��������������X�dz������ʵ���ҪԪ�أ���X��NԪ�أ�Y ԭ�ӵ������ֻ��2��������ԭ����������X������Y��MgԪ�أ�Z���ʿ��Ƴɰ뵼�������Z��SiԪ�أ�WԪ���γɵĵ���Ϊ��ɫ�Ĺ��壬��W��SԪ�أ�M�Ƕ���������Ԫ�أ���ԭ����������W������M��ClԪ�أ�
��1��W��SԪ�أ�SԪ�غ�����3�����Ӳ㣬�������6�����ӣ�Sԭ�ӵĻ�̬ԭ�ӵĺ�������Ų�ʽΪ��1s22s22p63s23p4��
�ʴ�Ϊ��1s22s22p63s23p4��
��2��X��NԪ�أ�Y��MgԪ�أ������γɵĻ�������Mg3N2�������Ӻ�þ����֮��������Ӽ���
�ʴ�Ϊ�����Ӽ���
��3��Z��SiԪ�أ��������辧�����乹������ԭ�ӣ���������Ϊ�ռ���״�ṹ������ԭ�Ӿ��壻���������д��ڵĻ�ѧ��ΪSi-O�������ڼ��Լ���
�ʴ�Ϊ��ԭ�Ӿ��壻���Լ���
��4��X��NԪ�ء�W��SԪ�أ�N��S�γɵ��⻯��ֱ�Ϊ���ң��Ҽס��������ĵ�������ȣ������N2H4������H2S�����൱�ڰ��������е�һ����ԭ�ӱ�����ȡ�������ԼĽṹʽΪ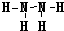 ��
��
�ʴ�Ϊ��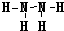 ��
��
��5��M��ClԪ�ء�W��SԪ�أ�ͬһ����Ԫ��ԭ�Ӱ뾶����ԭ���������������С����һ����������ԭ����������������������ƣ����Ե�һ�����ܽϴ��ΪCl��
�ʴ�Ϊ��Cl��
��1��W��SԪ�أ�SԪ�غ�����3�����Ӳ㣬�������6�����ӣ�Sԭ�ӵĻ�̬ԭ�ӵĺ�������Ų�ʽΪ��1s22s22p63s23p4��
�ʴ�Ϊ��1s22s22p63s23p4��
��2��X��NԪ�أ�Y��MgԪ�أ������γɵĻ�������Mg3N2�������Ӻ�þ����֮��������Ӽ���
�ʴ�Ϊ�����Ӽ���
��3��Z��SiԪ�أ��������辧�����乹������ԭ�ӣ���������Ϊ�ռ���״�ṹ������ԭ�Ӿ��壻���������д��ڵĻ�ѧ��ΪSi-O�������ڼ��Լ���
�ʴ�Ϊ��ԭ�Ӿ��壻���Լ���
��4��X��NԪ�ء�W��SԪ�أ�N��S�γɵ��⻯��ֱ�Ϊ���ң��Ҽס��������ĵ�������ȣ������N2H4������H2S�����൱�ڰ��������е�һ����ԭ�ӱ�����ȡ�������ԼĽṹʽΪ
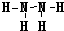 ��
���ʴ�Ϊ��
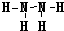 ��
����5��M��ClԪ�ء�W��SԪ�أ�ͬһ����Ԫ��ԭ�Ӱ뾶����ԭ���������������С����һ����������ԭ����������������������ƣ����Ե�һ�����ܽϴ��ΪCl��
�ʴ�Ϊ��Cl��
���������⿼����Ԫ��λ�ýṹ���ʵĹ�ϵ����ȷ�ƶ�Ԫ���ǽⱾ��ؼ����ѵ��ǵ���þ����ʽ����д������֪ʶǨ�Ƶķ�����д�µĽṹʽ��ע������ԭ�ӽṹ��Ԫ�����ڱ���Ԫ�������ɵĹ�ϵ����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
 ����VB2��-�������������ߵĵ�أ����ʾ��ͼ���£��õ�ع���ʱ��ӦΪ��4VB2+11O2��4B2O3+2V2O5������˵����ȷ���ǣ�������
����VB2��-�������������ߵĵ�أ����ʾ��ͼ���£��õ�ع���ʱ��ӦΪ��4VB2+11O2��4B2O3+2V2O5������˵����ȷ���ǣ�������| A���缫aΪ��ظ��� |
| B��ͼ��ѡ������Ĥֻ����������ѡ������ |
| C��������VB2����KOH��Һ����a�缫 |
| D��VB2�������ĵ缫��ӦΪ��2VB2+22OH--22e-��V2O5+2B2O3+11H2O |
�������ӷ���ʽ��ȷ���ǣ�������
| A�������ȥˮ���е�CaCO3��CaCO3+2H+=Ca2++H2O+CO2�� |
| B����ˮ��ͨ��������SO2��I2+SO2+2H2O=2HI+SO42-+2H+ |
| C��NaHSO4��Һ��Ba��OH��2��Һ��Ӧ�����ԣ�H++SO42-+Ba2++OH-=BaSO4��+H2O |
| D��4 mol?L-1��NaAlO2��Һ��7 mol?L-1��������Һ�������Ͼ���4AlO2-+7H++H2O=3A1��OH��3��+Al3+ |
��100mL BaCl2��KCl�Ļ����Һ�м���a mol Na2SO4��Һ��ǡ��ʹBa2+��ȫ�����������b mol AgNO3��Һ��ǡ��ʹCl-��ȫ��������û����Һ��K+Ũ��Ϊ��������
| A��0.1��2a-b��mol/L |
| B��0.1��b-2a��mol/L |
| C��10��b-2a��mol/L |
| D��10��2a-b��mol/L |
 +Cl2
+Cl2 +HCl ��R-CH2-CH=CH2+Cl2
+HCl ��R-CH2-CH=CH2+Cl2


