��Ŀ����
1�� ��֪��O2��g��+2H2��g��=2H2O��g����H=-483.6kJ•mol-1
��֪��O2��g��+2H2��g��=2H2O��g����H=-483.6kJ•mol-1C��s��ʯī��+O2��g��=CO2��g����H=-393.5kJ•mol-1
CO2��g��+C��s��ʯī��=2CO��g����H=+172.5kJ•mol-1
��1����д��CO��ˮ������Ӧ���Ȼ�ѧ����ʽCO��g��+H2O��g��?CO2��g��+H2��g����H=-41.2kJ•mol-1
��2����һ���Ϊ10L���ܱ������У�ͨ��һ������CO��ˮ��������850��ʱ������Ӧ��������CO��ˮ����Ũ�ȱ仯��ͼ����0��4min��ƽ����Ӧ����v��CO��=0.03mol/��L•min����������ʱ��ƽ�ⳣ��K=1��
| ʱ��/min | CO | H2O | CO2 | H2 |
| 0 | 0.200 | 0.300 | 0 | 0 |
| 2 | 0.138 | 0.238 | 0.062 | 0.062 |
| 3 | c1 | c2 | c3 | c3 |
| 4 | c1 | c2 | c3 | c3 |
| 5 | 0.116 | 0.216 | 0.084 | |
| 6 | 0.096 | 0.266 | 0.104 |
�ٱ���3min��4min֮�䷴Ӧ����ƽ��״̬��c1��ֵ����0.08mol/L ������ڡ�С�ڻ���ڣ���
�ڷ�Ӧ��4min��5min�䣬ƽ�����淽���ƶ������������µ�ԭ��d������ĸ��������5min��6min֮����ֵ�����仯�����ܵ�ԭ����a��������ĸ��
a������ˮ���� b�������¶� c��ʹ�ô��� d����������Ũ�ȣ�
���� ��1��O2��g��+2H2��g��=2H2O��g����H=-483.6kJ•mol-1��
C��s��ʯī��+O2��g��=CO2��g����H=-393.5kJ•mol-1��
CO2��g��+C��s��ʯī��=2CO��g����H=+172.5kJ•mol-1��
������ʽ$\frac{��-��-��}{2}$��CO��g��+H2O��g��?CO2��g��+H2��g����H������Ӧ�ĸı䣻
��2��0��4min��ƽ����Ӧ����v��CO��=$\frac{��c}{��t}$�����ݷ���ʽ֪��ƽ��ʱ��c��CO��=��c��CO2��=��c��H2��=��0.20-0.08��mol/L=0.12mol/L��c��CO��=0.08mol/L��c��H2O��=0.18mol/L����ѧƽ�ⳣ��K=$\frac{c��C{O}_{2}����c��{H}_{2}��}{c��CO����c��{H}_{2}O��}$��
��3���ٱ���3min-4min֮������ʵ�Ũ�Ȳ��䣬����ƽ��״̬��850��ﵽƽ��ʱc��CO��=0.08mol/L���÷�ӦΪ���ȷ�Ӧ�������¶������ƶ���
�ڷ�Ӧ��4min��5min��ƽ�����淽���ƶ�������ƽ���ƶ�ԭ�������ѡ���жϣ�
�ɱ������ݿ�֪��5min��6minCO��Ũ�Ƚ��ͣ�CO2Ũ������Ũ�ȱ仯����0.02mol/L��˵��ƽ��������Ӧ�ƶ����ٽ��ˮ��Ũ�ȱ仯�����жϣ�
��� �⣺��1��O2��g��+2H2��g��=2H2O��g����H=-483.6kJ•mol-1��
C��s��ʯī��+O2��g��=CO2��g����H=-393.5kJ•mol-1��
CO2��g��+C��s��ʯī��=2CO��g����H=+172.5kJ•mol-1��
������ʽ$\frac{��-��-��}{2}$��CO��g��+H2O��g��?CO2��g��+H2��g����H=$\frac{-393.5+483.6-172.5}{2}$kJ/mol=-41.2kJ•mol-1��
�ʴ�Ϊ��CO��g��+H2O��g��?CO2��g��+H2��g����H=-41.2kJ•mol-1��
��2��0��4min��ƽ����Ӧ����v��CO��=$\frac{��c}{��t}$=$\frac{0.20-0.08}{4}$mol/��L��min��=0.03 mol/��L•min�������ݷ���ʽ֪��ƽ��ʱ��c��CO��=��c��CO2��=��c��H2��=��0.20-0.08��mol/L=0.12mol/L��c��CO��=0.08mol/L��c��H2O��=0.18mol/L����ѧƽ�ⳣ��K=$\frac{c��C{O}_{2}����c��{H}_{2}��}{c��CO����c��{H}_{2}O��}$=$\frac{0.08��0.18}{0.12��0.12}$=1��
�ʴ�Ϊ��0.03 mol/��L•min����1��
��3�����ڸ���850��ʱ������Ӧ����ѧ��Ӧ���ʼӿ죬һ����4minǰ�ﵽ��ѧƽ�⣮����ӱ��пɿ�����Ӧ��3min��4minʱ�ĸ�����Ũ����ͬ����3min-4min֮�䷴ӦӦ����ƽ��״̬�������Ƿ��ȷ�Ӧ���¶����ߣ���ѧƽ�����淴Ӧ�����ƶ���C1��ֵӦ����0.08 mol/L��
�ʴ�Ϊ��ƽ�⣻���ڣ�
�ڷ�Ӧ��4min-5min�䣬ƽ�����淽���ƶ������������¶ȡ�����������Ũ�ȡ����ٷ�Ӧ��Ũ�ȵ��������𣬹�ѡd������5min-6min֮��COŨ�ȼ��٣�H2OŨ������CO2Ũ������ֻ������ˮ������ʹ��ѧƽ��������Ӧ�����ƶ�����ѡa��
�ʴ�Ϊ��d��a��
���� ���⿼�黯ѧƽ����㼰��������Ի�ѧƽ���ƶ���Ӱ�죬Ϊ��Ƶ���㣬���ؿ���ѧ������������������ͼ����Ϣ��ͼ����Ϣ���Ϸ�������ǽⱾ��ؼ�����Ŀ�Ѷ��еȣ�

 ̽���빮�̺��Ͽ�ѧ����������ϵ�д�
̽���빮�̺��Ͽ�ѧ����������ϵ�д�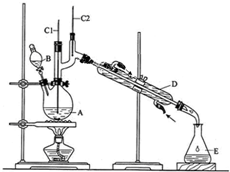
�����ķ�Ӧ���£�CH3CH2CH2CH2OH$��_{H_{2}SO_{4}/��}^{Na_{2}Cr_{2}O_{7}}$CH3CH2CH2CHO
��Ӧ��Ͳ������������б����£�
| �е�/�� | �ܶ�/g•cm-3 | ˮ���ܽ��� | |
| ������ | 117.2 | 0.8109 | �� |
| ����ȩ | 75.7 | 0.8017 | �� |
��6.0gNa2Cr2O7����100mL�ձ��У���30mLˮ�ܽ⣬�ٻ�������5mLŨ���ᣬ��������ҺС��ת����B�У���A�м���4.0g�������ͼ�����ʯ�����ȣ�������������ʱ����ʼ�μ�B����Һ���μӹ����б��ַ�Ӧ�¶�Ϊ90��95�棬��E���ռ�90�����ϵ���֣�������ﵹ���Һ©���У���ȥˮ�㣬�л������������ռ�75��77����֣�����2.0g���ش��������⣺
��1��ʵ���У��ܷ�Na2Cr2O7��Һ�ӵ�Ũ�����У�˵�����ɲ��ܣ��ױŽ���
��2�������ʯ�������Ƿ�ֹ���У�
��3������װ��ͼ�У�B�����������Ƿ�Һ©����D�����������������ܣ�
��4����Һ©��ʹ��ǰ������еIJ�����c������ȷ�𰸱�ţ���
a����ʪ b������ c����© d���궨
��5��������ȩ�ֲ�Ʒ���ڷ�Һ©���з�ˮʱ��ˮ���²㣨��ϡ����¡�����
��6����ʵ���У�����������ȩ�ļ��鷽�������������Dzⶨ��Է���������
| A�� | �٢ڢۢܢ� | B�� | �٢ۢݢܢ� | C�� | �٢ݢܢۢ� | D�� | �٢ڢݢۢ� |
| ��� | �� | �� | �� | �� |
| ����ʾ ��ͼ |  |  |  |  |
| �������� | �㵹Һ�� | ȡ�ÿ�״���� | ϡ��Ũ���� | ��������� |
| A�� | �� | B�� | �� | C�� | �� | D�� | �� |
| A�� | ��0.1 mol/L��ˮ��pHΪ11��NH3•H2O?NH4++OH- | |
| B�� | ˮ��ʯ����CO2+CaCO3+H2O�TCa��HCO3��2 | |
| C�� | NH4Cl�ܽ���T2O�У�NH4++T2O?NH3•T2O+H+ | |
| D�� | �����ȼ����Ϊ-1559.9 kJ•mol-1��������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ��2C2H6��g��+7O2��g���T4CO2��g��+6H2O��l����H=-3119.8kJ•mol-1 |
| A�� | 25�桢101KPaʱ��16g O2��O3��������к��е�ԭ����ΪNA | |
| B�� | 1 mol NH3�к��е�������Ϊ17NA | |
| C�� | 11.2 L O2��N2�Ļ�����庬�еķ�����Ϊ0.5NA | |
| D�� | ��������ϡ���ᷴӦ������0.1 mol H2ʱת�Ƶ�����Ϊ0.3NA |
| A�� | KԽ��Ӧ�ﵽƽ��ʱ���õ�ʱ��Խ�� | |
| B�� | KԽС���ﵽƽ��ʱ����Ӧ��� ת����Խ�� | |
| C�� | K�淴Ӧ��Ũ�ȸı���ı� | |
| D�� | KԽ�ﵽƽ��ʱ����Ӧ���еij̶�Խ�� |
| A�� | �ó���ʯ��ˮ����Na2CO3��NaHCO3��Һ | |
| B�� | ����ɫ��Ӧ����NaCl��KCl | |
| C�� | �ö����ЧӦ������������Һ���ὺ�� | |
| D�� | ����ˮ�������ռ�NO |

| ��Է������� | �ܶ�/��g•cm-3�� | �е�/�� | ˮ���ܽ��� | |
| ������ | 88 | 0.8123 | 131 | �� |
| ���� | 60 | 1.0492 | 118 | �� |
| ���������� | 130 | 0.8670 | 142 | ���� |
��A�м���4.4g���촼��6.0g���ᡢ����Ũ�����2��3Ƭ���Ƭ����ʼ��������A������50min����ӦҺ�������º����Һ©���У��ֱ�������ˮ������̼��������Һ��ˮϴ�ӣ��ֳ��IJ������������ˮMgSO4���壬����Ƭ�̣����˳�ȥMgSO4���壬�����������ռ�140��143����֣�������������3.9g��
�ش��������⣺
��1������B�����������������ܣ�
��2����ϴ�Ӳ����У���һ��ˮϴ����ҪĿ����ϴ��������ʹ��ᣬ�ڶ���ˮϴ����ҪĿ����ϴ��̼�����ƣ�
��3����ϴ�ӡ���Һ�����У�Ӧ�����Ȼ���ã����ֲ��d�����ţ���
a��ֱ�ӽ������������ӷ�Һ©�����Ͽڵ���
b��ֱ�ӽ������������ӷ�Һ©�����¿ڷų�
c���Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ��������������¿ڷų�
d���Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ��������������Ͽڵ���
��4����ʵ���м�����������Ŀ������ߴ���ת���ʣ�
��5��ʵ���м���������ˮMgSO4��Ŀ���Ǹ��
��6������������У�����ѡ��װ����ȷ����b�����ţ���
��7����ʵ��IJ�����c�����ţ���
a��30% ��b��40% c��60% d��90%
��8���ڽ����������ʱ������130��㿪ʼ�ռ���֣���ʹʵ��IJ���ƫ�ߣ���ߡ��͡�������ԭ���ǻ��ռ�����δ��Ӧ�����촼��
