��Ŀ����
8�� ���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�ã���ͼ��ʾһ�����أ�װ�е��Һa��X��Y������缫�壬ͨ��������ֱ����Դ��������ش��������⣺
���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�ã���ͼ��ʾһ�����أ�װ�е��Һa��X��Y������缫�壬ͨ��������ֱ����Դ��������ش��������⣺��1����Ҫ�õ�ⷽ��������ͭ�����Һaѡ��CuSO4��Һ����
��X�缫�IJ����Ǵ�ͭ��
�ڼ�����·����0.04mol����ͨ��ʱ����������1.28g��
��2����X��Y����Fe�缫��a�DZ���NaCl��Һ��ʵ�鿪ʼʱ��ͬʱ�����߸����뼸����ɫʯ����Һ����
�ٵ�����Y���ϵĵ缫��Ӧ��Fe-2e-=Fe2+��
����X�������۲쵽����������Һ���ɫ��
���� ��1����Ҫ�õ�ⷽ��������ͭ�����Һaѡ��CuSO4��Һ������Ӧ���Ǵ�ͭ�������Ǵ�ͭ����X�缫�Ǵ�ͭ��Y�缫�Ǵ�ͭ��������ͭ���ӷŵ磻����ת�Ƶ�����Cu�Ĺ�ϵ���㣻
��2����X��Y����Fe�缫��a�DZ���NaCl��Һ��XΪ�����������������ӵõ�������������ͬʱ�������������ӣ�YΪ������������Feʧ���������������ӣ�
��� �⣺��1������Ҫ�õ�ⷽ��������ͭ�����Һaѡ��CuSO4��Һ������Ӧ���Ǵ�ͭ�������Ǵ�ͭ����X�缫�Ǵ�ͭ��Y�缫�Ǵ�ͭ��
�ʴ�Ϊ����ͭ��
�ڼ�����·����0.04mol����ͨ��ʱ��������ͭ���ӷŵ磬�缫��ӦʽΪCu2++2e-=Cu�������ɵ�CuΪ0.02mol��������Ϊ0.02mol��64g/mol=1.28g��
�ʴ�Ϊ��1.28��
��2����X��Y����Fe�缫��a�DZ���NaCl��Һ��XΪ�����������������ӵõ�������������ͬʱ�������������ӣ�YΪ������������Feʧ���������������ӣ�
��YΪ������������Feʧ���������������ӣ���缫����ʽΪ��Fe-2e-=Fe2+���ʴ�Ϊ��Fe-2e-=Fe2+��
��XΪ�����������������ӵõ�������������ͬʱ�������������ӣ����������Լ��ԣ����뼸����ɫʯ����Һ����Һ���ɫ��
�ʴ�Ϊ����Һ���ɫ��
���� ���⿼��ѧ�����صĹ���ԭ��֪ʶ����ȷͼ�е�Դ��������ȷ�����ص��������ǽ��Ĺؼ�������Ϥ�缫��Ӧ�����ӵķŵ�˳���������Ŀ�Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д�| A�� |  �� �� | B�� | ���ʯ��ʯī | ||
| C�� | H3C-O-CH3��CH3CH2OH | D�� |  �� �� |
| A | B | C | D | |
| ���� | Zn | Fe | ̼�� | Cu |
| ���� | ̼�� | Zn | Zn | Zn |
| �������Һ | CuCl2 | H2SO4 | CuSO4 | ZnCl2 |
| A�� | A | B�� | B | C�� | C | D�� | D |

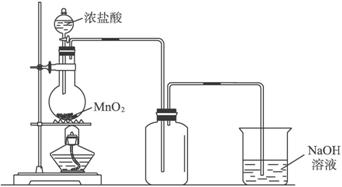
��1���Ʊ�����ѡ�õ�ҩƷΪ���������̺�Ũ���ᣬ��ط�Ӧ�Ļ�ѧ����ʽΪ��MnO2+4HCl��Ũ��$\frac{\underline{\;����\;}}{\;}$MnCl2+Cl2��+2H2O��
��2��װ��B�б���ʳ��ˮ�������dz�ȥCl2�е�HCl��ͬʱװ��B���ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������д����������ʱB�е�����B�г���©����Һ���������γ�ˮ����
��3��װ��F�ձ���ʢ�ŵ��Լ���NaOH��Һ�����з�����Ӧ�����ӷ���ʽ��2OH-+Cl2=Cl-+ClO-+H2O��
��4��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C�Т����η���D��
| ѡ�� | �� | �� | �� |
| A | �������ɫ���� | ��ʯ�� | ʪ�����ɫ���� |
| B | �������ɫ���� | �轺 | ʪ�����ɫ���� |
| C | ʪ�����ɫ���� | Ũ���� | �������ɫ���� |
| D | ʪ�����ɫ���� | ��ˮ�Ȼ��� | �������ɫ���� |

 ��仯ѧ���������о����̿���Ҫ�ɷ���MnO2���Ĺ����У�������Ũ�����ϼ��ȣ�������һ�ֻ���ɫ���̼�����ζ������--������ijѧ��ʹ����һԭ�������ͼ��ʾ��ʵ��װ�ã����������Ƶõ������볱ʪ����ʯ�ҷ�Ӧ��ȡ����Ư�ۣ�����һ�����ȷ�Ӧ�����ݴ˻ش��������⣺
��仯ѧ���������о����̿���Ҫ�ɷ���MnO2���Ĺ����У�������Ũ�����ϼ��ȣ�������һ�ֻ���ɫ���̼�����ζ������--������ijѧ��ʹ����һԭ�������ͼ��ʾ��ʵ��װ�ã����������Ƶõ������볱ʪ����ʯ�ҷ�Ӧ��ȡ����Ư�ۣ�����һ�����ȷ�Ӧ�����ݴ˻ش��������⣺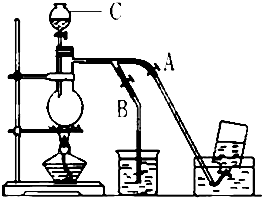 ��ͼ��ʾ��û��ͨ������������Ʊ�����ʱ��Ƶ�װ�ã�ͼ��a��b�ǿɿ��Ƶĵ������У��������ڱ����Ȼ�����Һ�е��ܽ�Ƚ�С����
��ͼ��ʾ��û��ͨ������������Ʊ�����ʱ��Ƶ�װ�ã�ͼ��a��b�ǿɿ��Ƶĵ������У��������ڱ����Ȼ�����Һ�е��ܽ�Ƚ�С����