��Ŀ����
3�����ʵĽṹ�������ʵ����ʣ���ش������漰���ʽṹ�����ʵ����⣺��1���ڶ������У�Ԫ�صĵ�һ�����ܴ���B��N֮���Ԫ����3�֣�
��2��ijԪ��λ�ڵ������ڢ��壬���̬ԭ�ӵ�δ�ɶԵ��������̬̼ԭ�ӵ�δ�ɶԵ�������ͬ�������̬ԭ�ӵļ۲�����Ų�ʽΪ3d84s2��
��3����ϩͪ��CH2=C=O����һ����Ҫ���л��м��壬����CH3COOH�ڣ�C2H5O��3P=O�����¼�����H2O�õ�����ϩͪ������̼ԭ���ӻ����������sp2��sp��1mol��C2H5O��3P=O�����к��еĦҼ�����ĿΪ25NA��
��4����֪��̬NH3��H2O��HF��������ܺͽṹ��ͼ1��
| ���� | ���X-H��Y | ����kJ��mol-1 |
| ��HF��n | D-H��F | 28.1 |
| �� | O-H��O | 18.8 |
| ��NH3��n | N-H��N | 5.4 |

����H2O��HF��NH3�е����ν��͵�ԭ������ļ����ǣ�HF��n��������NH3��n����ƽ��ÿ�����Ӻ������������2������HF��n�ͣ�NH3��nֻ��1��������Ҫ�˷���������ܼ����DZ�����HF��n����NH3��n����
��5��̼����Ľṹ����ʯ���ƣ���Ӳ�Ƚ����ڽ��ʯ�����н�ǿ����ĥ���ܣ�̼���辧���ṹ��ÿ��̼ԭ����Χ�����������Ĺ�ԭ����4������̼ԭ�ӵȾ��������̼ԭ����12������֪̼���辧���߳�Ϊapm����ͼ2��1�Ź�ԭ�Ӻ�2��̼ԭ��֮��ľ���Ϊ$\frac{\sqrt{11}a}{4}$pm��̼������ܶ�Ϊ$\frac{1.6��1{0}^{32}}{{a}^{3}��{N}_{A}}$g/cm3��
���� ��1��ͬһ����Ԫ���У�Ԫ�صĵ�һ����������ԭ����������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ���ͬ��������Ԫ�صģ�
��2��ijԪ��λ�ڵ������ڢ��壬���̬ԭ�ӵ�δ�ɶԵ��������̬̼ԭ�ӵ�δ�ɶԵ�������ͬ��̼ԭ�ӵĵ����Ų�Ϊ1s22s22p2��δ�ɶԵ�����Ϊ2�����Ԫ��ΪNi��
��3����ϩͪ������̼ԭ�Ӿ�û�й¶Ե��ӣ�CH2��Cԭ���γ�3���Ҽ�����C=O��̼ԭ���γ�2���Ҽ����ӻ������Ŀ�ֱ�Ϊ3��2����C2H5O��3P=O���Ӻ���25���Ҽ���
��4��ƽ��ÿ�����Ӻ������������2������HF��n�ͣ�NH3��nֻ��1�����������Ҫ�˷���������ܼ��ܣ�
��5�����ݾ�̯�����㾧����Si��Cԭ����Ŀ��ÿ��Siԭ����Χ��4��̼ԭ�ӣ�ԭ����λ����ԭ����Ŀ�ɷ��ȣ����Լ���̼ԭ����Χ�����������Ĺ�ԭ����Ŀ��
�Զ���Cԭ���о�����֮���������Cԭ��λ�������ϣ�ÿ������ԭ��Ϊ8���������ã�ÿ����Ϊ2���������ã�
����1��Siԭ�ӵ���Խ��ߡ�2��̼ԭ�ӵ���Խ��ߣ��ཻ��O�㣬�붥��̼ԭ���γ���ͼ��ʾ��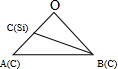 ������BΪ2��̼ԭ�ӣ�CΪ1��Siԭ�ӣ�1��Siԭ������Χ��4��Cԭ���γ��������壬1��Siԭ���붥��̼ԭ�����ߴ��ھ�����Խ����ϣ��Ҿ���Ϊ��Խ��߳��ȵ�$\frac{1}{4}$����Խ��߳���Ϊ$\sqrt{3}$a pm����OA=OB=$\frac{\sqrt{3}}{2}$a pm����OC=$\frac{\sqrt{3}}{4}$a pm���������Ҷ�������cos��AOB��ֵ�����������Ҷ�������BC�ij��ȣ�������ͼ2��1�Ź�ԭ�Ӻ�2��̼ԭ��֮��ľ��룻
������BΪ2��̼ԭ�ӣ�CΪ1��Siԭ�ӣ�1��Siԭ������Χ��4��Cԭ���γ��������壬1��Siԭ���붥��̼ԭ�����ߴ��ھ�����Խ����ϣ��Ҿ���Ϊ��Խ��߳��ȵ�$\frac{1}{4}$����Խ��߳���Ϊ$\sqrt{3}$a pm����OA=OB=$\frac{\sqrt{3}}{2}$a pm����OC=$\frac{\sqrt{3}}{4}$a pm���������Ҷ�������cos��AOB��ֵ�����������Ҷ�������BC�ij��ȣ�������ͼ2��1�Ź�ԭ�Ӻ�2��̼ԭ��֮��ľ��룻
��Ͼ�����ԭ����Ŀ����ʾ�������������ٸ��ݦ�=$\frac{m}{V}$���㾧���ܶȣ�
��� �⣺��1��ͬһ����Ԫ���У�Ԫ�صĵ�һ����������ԭ����������������������ƣ��������Nԭ�Ӻ�ȫ������Beԭ�ӵ�һ������Ҫ��ͬ��������Ԫ�صĸߣ��ʵ�һ�����ܽ���B��N֮��ĵڶ�����Ԫ����Be��C��O����Ԫ�أ�
�ʴ�Ϊ��3��
��2��ijԪ��λ�ڵ������ڢ��壬���̬ԭ�ӵ�δ�ɶԵ��������̬̼ԭ�ӵ�δ�ɶԵ�������ͬ��Cԭ�ӵĵ����Ų�Ϊ1s22s22p2��δ�ɶԵ�����Ϊ2�����Ԫ��ΪNi�����̬ԭ�ӵļ۲�����Ų�ʽΪ3d84s2��
�ʴ�Ϊ��3d84s2��
��3����ϩͪ������̼ԭ�Ӿ�û�й¶Ե��ӣ�CH2��Cԭ���γ�3���Ҽ�����C=O��̼ԭ���γ�2���Ҽ����ӻ������Ŀ�ֱ�Ϊ3��2������̼ԭ�ӵ��ӻ����������sp2��sp����C2H5O��3P=O���Ӻ���25���Ҽ���1mol��C2H5O��3P=O�����к��еĦҼ�����ĿΪ25NA��
�ʴ�Ϊ��sp2��sp��25NA��
��4����������ļ����ǣ�HF��n��������NH3��n����ƽ��ÿ�����Ӻ������������2������HF��n�ͣ�NH3��nֻ��1��������Ҫ�˷���������ܼ����DZ�����HF��n����NH3��n����H2O��HF��NH3�е����ν��ͣ�
�ʴ�Ϊ����������ļ����ǣ�HF��n��������NH3��n����ƽ��ÿ�����Ӻ������������2������HF��n�ͣ�NH3��nֻ��1��������Ҫ�˷���������ܼ����DZ�����HF��n����NH3��n��
��5��������Siԭ����ĿΪ4��Cԭ����ĿΪ8��$\frac{1}{8}$+6��$\frac{1}{2}$=4��ÿ��Siԭ����Χ��4��̼ԭ�ӣ�ԭ����λ����ԭ����Ŀ�ɷ��ȣ���̼ԭ����λ��Ҳ��4����̼ԭ����Χ�����������Ĺ�ԭ����ĿΪ4��
�Զ���Cԭ���о�����֮���������Cԭ��λ�������ϣ�ÿ������ԭ��Ϊ8���������ã�ÿ����Ϊ2���������ã���̼ԭ�ӵȾ��������̼ԭ����$\frac{8��3}{2}$=12����
����1��Siԭ�ӵ���Խ��ߡ�2��̼ԭ�ӵ���Խ��ߣ��ཻ��O�㣬�붥��̼ԭ���γ���ͼ��ʾ�� ������BΪ2��̼ԭ�ӣ�CΪ1��Siԭ�ӣ�1��Siԭ������Χ��4��Cԭ���γ��������壬1��Siԭ���붥��̼ԭ�����ߴ��ھ�����Խ����ϣ��Ҿ���Ϊ��Խ��߳��ȵ�$\frac{1}{4}$����Խ��߳���Ϊ$\sqrt{3}$a pm����OA=OB=$\frac{\sqrt{3}}{2}$a pm����OC=$\frac{\sqrt{3}}{4}$a pm����
������BΪ2��̼ԭ�ӣ�CΪ1��Siԭ�ӣ�1��Siԭ������Χ��4��Cԭ���γ��������壬1��Siԭ���붥��̼ԭ�����ߴ��ھ�����Խ����ϣ��Ҿ���Ϊ��Խ��߳��ȵ�$\frac{1}{4}$����Խ��߳���Ϊ$\sqrt{3}$a pm����OA=OB=$\frac{\sqrt{3}}{2}$a pm����OC=$\frac{\sqrt{3}}{4}$a pm����
��$\frac{\sqrt{3}}{2}$a��2+��$\frac{\sqrt{3}}{2}$a��2-2��$\frac{\sqrt{3}}{2}$a��$\frac{\sqrt{3}}{2}$a��cos��AOB=a2��
���cos��AOB=$\frac{1}{3}$
�ʣ�$\frac{\sqrt{3}}{4}$a��2+��$\frac{\sqrt{3}}{2}$a��2-2��$\frac{\sqrt{3}}{4}$a��$\frac{\sqrt{3}}{2}$a��$\frac{1}{3}$=BC2��
���BC=$\frac{\sqrt{11}a}{4}$
��������Ϊ4��$\frac{28+12}{{N}_{A}}$g�������ܶ�Ϊ4��$\frac{28+12}{{N}_{A}}$g�£�a��10-10 cm��3=$\frac{1.6��1{0}^{32}}{{a}^{3}��{N}_{A}}$g/cm3��
�ʴ�Ϊ��4��12��$\frac{\sqrt{11}a}{4}$��$\frac{1.6��1{0}^{32}}{{a}^{3}��{N}_{A}}$��
���� �����Ƕ����ʽṹ�����ʵĿ��飬�漰��������Ų��������ܡ��ӻ��������ѧ�����������������ȣ���4����5��Ϊ�״��㡢�ѵ㣬���ؿ���ѧ�����������������ѶȽϴ�

| A�� | ��FeCl3��Һ�еμӹ�����ˮ������ȡFe��OH��3���� | |
| B�� | ȡ������ҺX�������м����������Ƶ���ˮ���ټӼ���KSCN��Һ����Һ��죬˵��X��Һ��һ������Fe2+ | |
| C�� | ����������ȥ�����л��е�NaCl | |
| D�� | ��֪${I}_{3}^{-}$?I2+I-����ʢ��KI3��Һ���Թ��м�������CCl4�����ú�CCl4������ɫ��˵��KI3��CCl4�е��ܽ�ȱ���ˮ�еĴ� |
| A�� | I��Br��Cl | B�� | Al��P��Si | C�� | O��S��Na | D�� | N��C��B |
| A�� | ${\;}_{62}^{144}$Sm��${\;}_{62}^{150}$Sm��Ϊͬλ�� | |
| B�� | ${\;}_{62}^{144}$Sm��${\;}_{62}^{150}$Sm����������ͬ | |
| C�� | ${\;}_{62}^{144}$Sm��${\;}_{62}^{150}$Sm��ͬһ�ֺ��� | |
| D�� | ${\;}_{62}^{144}$Sm��${\;}_{62}^{150}$Sm�Dz�ͬԪ�� |
| A�� | �����£���ˮ�������c��H+��=10-12mol/L������ҺpHһ��Ϊ12 | |
| B�� | ��֪H2C2O4�����ᣬ����뷽��ʽΪ��H2C2O4?2H++C2O42- | |
| C�� | �����£�pH=10�İ�ˮ��Һ�У���ˮ�������c��H+��=10-10mol/L | |
| D�� | ��ҵ�ϳɰ��¶ȿ�����500�棬Ŀ����Ϊ����߲��� |


