��Ŀ����
��1����Ϊ�ڢ�A��Ԫ�أ����ĵ��ʺͻ�������ijЩ���ʵĻ�ѧ��������������֮������֪��Ԫ�ؾ����������ʣ�
Sn4++Sn=2Sn2++O2+4H+=2Sn4++2H2O��
2H++SnO22-?Sn��OH��2?Sn2++2OH-��
�Իش�
�����������ᣬ����Ӧ�����Һ��ͨ���������йط�Ӧ������������Ӧ�仯����д���йط�Ӧ�Ļ�ѧ����ʽ�� �� ��
�ڽ�������Һ���ɺ�����������ù��壬�仯����������FeCl3��Һ��Ӧ�ı仯�������õ��Ĺ��������� ���ѧʽ����
��������SnCl2��Һ������ļ���Һ��Ӧ�ķ�����Sn��OH��2���ü��ѡ�� ��
��2��ij��ɫϡ��ҺX�У����ܺ����±����������е�ij���֣�
��3����ȡ����Һ�����������м���ij�Լ�Y���������������ʵ�����n��������Լ�Y�����V���Ĺ�ϵ��ͼ��ʾ��
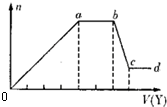 ����Y�����ᣬ����Һ�к��еĽ����������� ��ab�η�����Ӧ�����ӷ���ʽΪ ��ͼ��Oa�βμӷ�Ӧ�������ӵ����ʵ���֮��Ϊ ��
����Y�����ᣬ����Һ�к��еĽ����������� ��ab�η�����Ӧ�����ӷ���ʽΪ ��ͼ��Oa�βμӷ�Ӧ�������ӵ����ʵ���֮��Ϊ ��
����Y��NaOH��Һ����bc�η�Ӧ�����ӷ���ʽΪ ��
�����������ӵ�ˮ�⣬����H+��OH-��Ӱ�죬����Һ��ֻ�����������ӣ������ǵ����Ӹ�����Ϊ ������������ǰ���������ں���ǰ���ͼ��ں��˳�����У���
Sn4++Sn=2Sn2++O2+4H+=2Sn4++2H2O��
2H++SnO22-?Sn��OH��2?Sn2++2OH-��
�Իش�
�����������ᣬ����Ӧ�����Һ��ͨ���������йط�Ӧ������������Ӧ�仯����д���йط�Ӧ�Ļ�ѧ����ʽ��
�ڽ�������Һ���ɺ�����������ù��壬�仯����������FeCl3��Һ��Ӧ�ı仯�������õ��Ĺ���������
��������SnCl2��Һ������ļ���Һ��Ӧ�ķ�����Sn��OH��2���ü��ѡ��
��2��ij��ɫϡ��ҺX�У����ܺ����±����������е�ij���֣�
| ������ | CO32-��SiO32-��AlO2-��Cl- |
| ������ | Al3+��Cu2+��Mg2+��NH4+��Na+ |
 ����Y�����ᣬ����Һ�к��еĽ�����������
����Y�����ᣬ����Һ�к��еĽ���������������Y��NaOH��Һ����bc�η�Ӧ�����ӷ���ʽΪ
�����������ӵ�ˮ�⣬����H+��OH-��Ӱ�죬����Һ��ֻ�����������ӣ������ǵ����Ӹ�����Ϊ
���㣺���ӷ���ʽ���йؼ���,̼��Ԫ�ؼ��
ר�⣺������,Ԫ�ؼ��仯����
��������1�����������ᷴӦ���ɶ��Ȼ��������Ȼ��������������������Ȼ�����
�ڽ��Ȼ�����Һ�������ù��������ڸ���������ݶ��ߵ�������֪�����Ȼ�����Һ���ɵõ�������������
�۸��������Ϣ֪��Sn��OH��2�������ԣ��ܺ�ǿ�Ӧ��������ȡ������ʱӦ�������
��3����Һ��ɫ˵����Һ�в���ͭ���ӣ�
�����Y�����ᣬ����Һ�м����ᣬ�����ɳ�������a-b��ʱ�������������仯�������̼������ӷ�Ӧ�������壬����Һ�в���þ���ӡ������ӣ���b-c��ʱ�������������٣����ֳ��������ᷴӦ�����ֳ����������Ӧ��˵����Һ���й�������Ӻ�ƫ��������ӣ���������Ӻ�笠�������˫ˮ�⣬������Һ�к��е��������������ӣ�
����Y���������ƣ�����Һ�м�����������Һ�������ɳ�������a-b��ʱ�������������仯���������ƺ�笠����ӷ�Ӧ�������壻��b-c��ʱ�������������٣����ֳ������������Ʒ�Ӧ�����ֳ�������Ӧ��˵����Һ�����������Ӻ�þ���ӣ�����Һ�в�����������ӡ�̼������Ӻ�ƫ��������ӣ�������Һ�к��е��������������ӣ�
�ڽ��Ȼ�����Һ�������ù��������ڸ���������ݶ��ߵ�������֪�����Ȼ�����Һ���ɵõ�������������
�۸��������Ϣ֪��Sn��OH��2�������ԣ��ܺ�ǿ�Ӧ��������ȡ������ʱӦ�������
��3����Һ��ɫ˵����Һ�в���ͭ���ӣ�
�����Y�����ᣬ����Һ�м����ᣬ�����ɳ�������a-b��ʱ�������������仯�������̼������ӷ�Ӧ�������壬����Һ�в���þ���ӡ������ӣ���b-c��ʱ�������������٣����ֳ��������ᷴӦ�����ֳ����������Ӧ��˵����Һ���й�������Ӻ�ƫ��������ӣ���������Ӻ�笠�������˫ˮ�⣬������Һ�к��е��������������ӣ�
����Y���������ƣ�����Һ�м�����������Һ�������ɳ�������a-b��ʱ�������������仯���������ƺ�笠����ӷ�Ӧ�������壻��b-c��ʱ�������������٣����ֳ������������Ʒ�Ӧ�����ֳ�������Ӧ��˵����Һ�����������Ӻ�þ���ӣ�����Һ�в�����������ӡ�̼������Ӻ�ƫ��������ӣ�������Һ�к��е��������������ӣ�
���
�⣺��1�����������ᷴӦ���ɶ��Ȼ��������Ȼ��������������������Ȼ�������Ӧ����ʽΪ��Sn+2HCl=SnCl2+H2����SnCl2+Cl2=SnCl4��
�ʴ�Ϊ��Sn+2HCl=SnCl2+H2���� SnCl2+Cl2=SnCl4
�ڽ��Ȼ�����Һ�������ù��������ڸ���������ݶ��ߵ�������֪�����Ȼ�����Һ���ɵõ��������������������õ��Ĺ�����SnO2���ʴ�Ϊ��SnO2��
�۸��������Ϣ֪��Sn��OH��2�������ԣ��ܺ�ǿ�Ӧ��������ȡ������ʱӦ��������������ΪNH3?H2O���ʴ�Ϊ��NH3?H2O��
��3����Һ��ɫ˵����Һ�в���ͭ���ӣ�
�����Y�����ᣬ����Һ�м����ᣬ�����ɳ�������a-b��ʱ�������������仯�������̼������ӷ�Ӧ�������壬����Һ�в���þ���ӡ������ӣ���b-c��ʱ�������������٣����ֳ��������ᷴӦ�����ֳ����������Ӧ��˵����Һ���й�������Ӻ�ƫ��������ӣ���������Ӻ�笠�������˫ˮ�⣬������Һ�к��е��������������ӣ�ͨ�����Ϸ���֪����Һ�к��е��������������ӣ�ab�η�����Ӧ��̼������Ӻ������ӷ�Ӧ���ɶ�����̼��ˮ�����ӷ���ʽΪ��CO32-+2H+=H2O+CO2��������ͼ��֪���������������ᷴӦ��Ҫ1V���ᣬƫ��������Ӻ�������Ӻ����ᷴӦ��Ҫ����4V���ᣬ�йط�Ӧ����ʽΪ��AlO2-+H++H2O=Al��OH��3����SiO32-+2H+=H2SiO3 ����Al��OH��3+3H+=Al3++3H2O�����ݷ���ʽ֪��SiO32-����H2SiO3��Ҫ����������AlO2-����Al��OH��3 ��Ҫ��������֮��
=11��1������AlO2-+H++H2O=Al��OH��3����SiO32-+2H+=H2SiO3 ��֪���� n��SiO32-����n ��AlO2-��=
��
=11��2��
�ʴ�Ϊ��Na+��CO32-+2H+=H2O+CO2����n��SiO32-����n ��AlO2-��=11��2��
����Y���������ƣ�����Һ�м�����������Һ�������ɳ�������a-b��ʱ�������������仯���������ƺ�笠����ӷ�Ӧ�������壻��b-c��ʱ�������������٣����ֳ������������Ʒ�Ӧ�����ֳ�������Ӧ��˵����Һ�����������Ӻ�þ���ӣ�����Һ�в�����������ӡ�̼������Ӻ�ƫ��������ӣ�������Һ�к��е��������������ӣ�
Y��NaOH��Һ����bc���������������������Ʒ�Ӧ����ƫ�����ƺ�ˮ�����ӷ���ʽΪ��Al��OH��3+OH-=AlO2-+2H2O��
��笠�������Ҫ�������Ƶ������2V�������������������Ʒ�Ӧ��Ҫ�������Ƶ������1V������������������Ҫ�������Ƶ������3V������������þ��Ҫ�������Ƶ������1V����n��Al3+����n��Mg2+����n��NH4+��=1��
��2=2��1��4����Һ��������������������֪��n��Al3+����n��Mg2+ ����n��NH4+����n��Cl- ��=2��1��4��12����N��Al3+����N��Mg2+ ����N��NH4+����N��Cl- ��=2��1��4��12��
�ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��N��Al3+����N��Mg2+ ����N��NH4+����N��Cl- ��=2��1��4��12��
�ʴ�Ϊ��Sn+2HCl=SnCl2+H2���� SnCl2+Cl2=SnCl4
�ڽ��Ȼ�����Һ�������ù��������ڸ���������ݶ��ߵ�������֪�����Ȼ�����Һ���ɵõ��������������������õ��Ĺ�����SnO2���ʴ�Ϊ��SnO2��
�۸��������Ϣ֪��Sn��OH��2�������ԣ��ܺ�ǿ�Ӧ��������ȡ������ʱӦ��������������ΪNH3?H2O���ʴ�Ϊ��NH3?H2O��
��3����Һ��ɫ˵����Һ�в���ͭ���ӣ�
�����Y�����ᣬ����Һ�м����ᣬ�����ɳ�������a-b��ʱ�������������仯�������̼������ӷ�Ӧ�������壬����Һ�в���þ���ӡ������ӣ���b-c��ʱ�������������٣����ֳ��������ᷴӦ�����ֳ����������Ӧ��˵����Һ���й�������Ӻ�ƫ��������ӣ���������Ӻ�笠�������˫ˮ�⣬������Һ�к��е��������������ӣ�ͨ�����Ϸ���֪����Һ�к��е��������������ӣ�ab�η�����Ӧ��̼������Ӻ������ӷ�Ӧ���ɶ�����̼��ˮ�����ӷ���ʽΪ��CO32-+2H+=H2O+CO2��������ͼ��֪���������������ᷴӦ��Ҫ1V���ᣬƫ��������Ӻ�������Ӻ����ᷴӦ��Ҫ����4V���ᣬ�йط�Ӧ����ʽΪ��AlO2-+H++H2O=Al��OH��3����SiO32-+2H+=H2SiO3 ����Al��OH��3+3H+=Al3++3H2O�����ݷ���ʽ֪��SiO32-����H2SiO3��Ҫ����������AlO2-����Al��OH��3 ��Ҫ��������֮��
4-
| ||
|
4-
| ||
| 2 |
| 1 |
| 3 |
�ʴ�Ϊ��Na+��CO32-+2H+=H2O+CO2����n��SiO32-����n ��AlO2-��=11��2��
����Y���������ƣ�����Һ�м�����������Һ�������ɳ�������a-b��ʱ�������������仯���������ƺ�笠����ӷ�Ӧ�������壻��b-c��ʱ�������������٣����ֳ������������Ʒ�Ӧ�����ֳ�������Ӧ��˵����Һ�����������Ӻ�þ���ӣ�����Һ�в�����������ӡ�̼������Ӻ�ƫ��������ӣ�������Һ�к��е��������������ӣ�
Y��NaOH��Һ����bc���������������������Ʒ�Ӧ����ƫ�����ƺ�ˮ�����ӷ���ʽΪ��Al��OH��3+OH-=AlO2-+2H2O��
��笠�������Ҫ�������Ƶ������2V�������������������Ʒ�Ӧ��Ҫ�������Ƶ������1V������������������Ҫ�������Ƶ������3V������������þ��Ҫ�������Ƶ������1V����n��Al3+����n��Mg2+����n��NH4+��=1��
| 1 |
| 2 |
�ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��N��Al3+����N��Mg2+ ����N��NH4+����N��Cl- ��=2��1��4��12��
���������⿼����������ƶϼ����ʵ������ԣ�������Һ����ɫ������ͼ��ȷ����Һ�д��ڵ����ӣ��ٽ������֮��ķ�Ӧ��ȷ����������ͬʱ����ѧ��˼ά�������ԡ����������ȫ���ԣ��ڵ�һ���У�����֪ʶǨ�Ƶķ�������������Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
50gþ��п�����Ļ������������ϡ���ᷴӦ�õ������Һ�������õ�218g���壨����Ϊ��ˮ�����Σ�����Ӧ���������������ǣ�������
| A��3.5g | B��3g |
| C��2g | D��4.5g |
ij�ܱ������г�������ʵ���������A��B��һ���¶��·�����Ӧ��A��g��+xB��g��?2C��g�����ﵽƽ���ֻ�ı䷴Ӧ��һ��������������������ʵ�Ũ�ȡ���Ӧ������ʱ��ı仯��ͼ��ʾ������˵������ȷ�ǣ�������


| A��30 min��40 min��÷�Ӧʹ���˴��� |
| B����Ӧ����ʽ�е�x=1������ӦΪ���ȷ�Ӧ |
| C��30��40min�ı�������ǽ����������������ԭ����2�� |
| D����25min��35min��55minʱ��ѧƽ�ⳣ���ֱ�ΪK1��K2��K3����K1=K2��K3 |
��CO2��H2��CO��ɵĻ������ͬ��ͬѹ���뵪�����ܶ���ͬ����û��������CO2��H2��CO������Ȳ�����Ϊ��������
| A��39��24��13 |
| B��22��7��14 |
| C��13��8��29 |
| D��26��16��57 |
��NAΪ�����ӵ�����������˵���У���ȷ���ǣ�������
| A��2.7g����������������ĿΪ0.1 NA |
| B��16 g CH4����ԭ����ĿΪNA |
| C��17 g NH3������������ĿΪNA |
| D��18 gˮ����������ĿΪNA |
���й����л������ȷ˵���ǣ�������
| A������ϩ�ɷ����ӳɷ�Ӧ |
| B������Ķ��ȴ�����9��ͬ���칹�� |
| C�����ۡ���������ȫˮ��IJ��ﻥΪͬ���칹�� |
| D����ά�غ���֬���Ǹ߷��ӻ����� |
����˵����ȷ���ǣ�������
| A������Ǽ� |
| B��ʳ�β����� |
| C�����Ǽ� |
| D���ɱ����DZ� |
 �±���Ԫ�����ڱ���һ���֣�����������ĸ�ֱ����ijһ��ѧԪ�أ�
�±���Ԫ�����ڱ���һ���֣�����������ĸ�ֱ����ijһ��ѧԪ�أ� ʵ������NaOH��FeSO4��ȡFe��OH��2ʱ��Fe��OH��2�����ױ���������������ͼ��ʾװ����ȡ�����Եõ��ϴ�����Fe��OH��2��
ʵ������NaOH��FeSO4��ȡFe��OH��2ʱ��Fe��OH��2�����ױ���������������ͼ��ʾװ����ȡ�����Եõ��ϴ�����Fe��OH��2��