��Ŀ����
19�� ijѧ����0.1mol•L-1��KOH����Һ�ζ�δ֪Ũ�ȵ����ᣬ������ֽ�Ϊ���¼�����
ijѧ����0.1mol•L-1��KOH����Һ�ζ�δ֪Ũ�ȵ����ᣬ������ֽ�Ϊ���¼�����A������ʽ�ζ���ȷ��ȡ20mL��������ע��ྻ����ƿ��������2��3��ָʾ��
B���ñ���Һ������������Һ�ֱ���ϴ��ʽ�ζ��ܡ���ʽ�ζ���2��3��
C����ʢ�б���Һ�ļ�ʽ�ζ��̶ܹ��ã����ڵζ��ܼ���ʹ֮������Һ
D��ȡ��KOH��Һע���ʽ�ζ�������0���̶�����1��2cm
E������Һ������0����0�����¿̶ȣ����¶���
F������ƿ�µ�һ�Ű�ֽ������ƿ���ڵζ��ܵ����棬�ñ�KOH��Һ�ζ����յ㲢���µζ���Һ��Ŀ̶ȣ��ʹ�ʵ�����������գ�
��1����ȷ���������˳����BDCEAF���������ĸ��д����
��2�����п�����Ϊ���εζ�ʵ�����ָʾ������C��
A������ B��ʯ�� C����̪ D��KMnO4
��3���жϵ���ζ��յ��ʵ�������ǵ������һ��KOH��Һ����Һ����ɫ��Ϊdz��ɫ�����ڰ�����ڲ���ɫ��
��4����ͼ��ʾij�εζ�ʱ50mL�ζ�����ǰ��Һ���λ�ã��뽫��ȥ�ı�KOH��Һ����������±��ո��У��й����ݼ�¼���£�
| �ζ���� | ����Һ�����mL�� | ������KOH��Һ�������mL�� | ||
| �ζ�ǰ | �ζ��� | ���ĵ���� | ||
| 1 | 20.00 | 0.50 | 25.12 | 24.62 |
| 2 | 20.00 | 0.30 | 24.90 | 24.60 |
| 3 | 20.00 | 6.00 | 30.58 | 24.58 |
��6�����м��������ʹ�ⶨ���ƫ�ߵ���ac��
a������A�������֮ǰ�����ô���Һ��ϴ��ƿ
b������ʱ�����ζ�ǰ���ӣ��ζ�����
c�����ڵζ������в��������μ�Һ������ƿ��
d����δ������տ�����Һ��ɫ������ֹͣ�ζ�
f������ȡһ������KOH���壨������NaOH�����Ʊ���Һ�������ζ��������ᣮ
���� ��1���к͵ζ��м�©��ϴ�ӡ���ϴ��װҺ��ȡ����Һ����ƿ��Ȼ�����ָʾ�����еζ��Ȳ�����
��2����0.1mol•L-1��KOH����Һ�ζ�δ֪Ũ�ȵ�����ǡ����ȫ��Ӧ��Һ�����ԣ�����������Һ����ָʾ�յ�������DZ��ɫ��ѡ���ָ̪ʾ����
��3������Һ��ɫ�仯�Ұ�����ڲ���ɫ����˵���ﵽ�ζ��յ㣻
��4����ϵζ��ܵĽṹ�Լ����������ԭ������ȡ�������밼Һ����ʹ���ƽ��ȡ�ζ��ܵ����ݼ������ı���Һ�����
��5���ȿ������ݵĺ����ԣ�Ȼ���������ƽ��ֵ��Ȼ�����c�����⣩=$\frac{c������V������}{V�����⣩}$���㣻
��6������c�����⣩=$\frac{c������V������}{V�����⣩}$������
��� �⣺��1�������IJ�����ѡ��ζ��ܣ�Ȼ��ϴ�ӡ�װҺ��ʹ���������Һ���̶��ڵζ�̨�ϣ�Ȼ�����Һ����¶�������ȡ����Һ����ƿ��Ȼ�����ָʾ�����еζ�������˳��Ϊ��B��D��C��E��A��F��
�ʴ�Ϊ��BDCEAF��
��2����0.1mol•L-1��KOH����Һ�ζ�δ֪Ũ�ȵ�����ǡ����ȫ��Ӧ��Һ�����ԣ�����������Һ����ָʾ�յ�������DZ��ɫ��ѡ���ָ̪ʾ�������۲���ָʾ��Ӧ�յ㣬���������Һ�������������������Һ��Ӧ��ʯ���Լ���ɫ��Χ������ָʾ����
�ʴ�Ϊ��C��
��3����ʵ������NaOH�ζ�������Һ���÷�̪��ָʾ���������յ�ʱ�������ǵ���Һ����ɫ��Ϊdz��ɫ�����ڰ�����ڲ���ɫ��
�ʴ�Ϊ���������һ��NaOH��Һ����Һ����ɫ��Ϊdz��ɫ�����ڰ�����ڲ���ɫ��
��4���ζ�ǰ����Ϊ0.30mL���ζ������Ϊ24.90mL��������Һ�����Ϊ24.60mL���ʴ�Ϊ��0.30��24.90��24.60��
��5�������1�顢��2�顢��3�����ĵ������Һ�����Ϊ��$\frac{24.58+24.60+24.62}{3}$mL=24.60mL��c�����⣩=$\frac{24.60ml��0.1mol/L}{20.00ml}$=$\frac{24.60��0.1}{20.00}$mol/L��
�ʴ�Ϊ��$\frac{24.60��0.1}{20.00}$mol/L��
��6��a����ƿˮϴ��ֱ��װ����Һ�����ĵı�Һ�����Ӱ�죬���ô���Һ��ϴ��ƿ���ⶨ���ƫ�ߣ���a��ȷ��
b���ζ��ܶ���ʱ�����ζ�ǰ���ӣ��ζ����ӣ�����V������ƫС����c�����⣩=$\frac{c������V������}{V�����⣩}$����������c�����⣩ƫ�ͣ���b����
c�����ڵζ������в��������μ�Һ������ƿ�⣬����V������ƫ��c�����⣩=$\frac{c������V������}{V�����⣩}$����������c�����⣩ƫ�ߣ���c��ȷ��
d����δ������տ�����Һ��ɫ������ֹͣ�ζ������ı���Һ���ƫС����c�����⣩=$\frac{c������V������}{V�����⣩}$����������c�����⣩ƫ�ͣ���d����
f������ȡһ������KOH���壨������NaOH�����Ʊ���Һ�������ζ��������ᣬ�����������ʵ����������ı���Һ�����С����c�����⣩=$\frac{c������V������}{V�����⣩}$����������c�����⣩ƫ�ͣ���f����
�ʴ�Ϊ��ac��
���� ������Ҫ�������к͵ζ��������������жϡ�����ʹ�õȣ��Ѷ��еȣ��������ʱҪ���Ƿ�Ӱ���������������������ƫ���ƫ�ߣ��������ƫС������ƫС������Ӱ��������������Ӱ�죮

| A�� | Ksp��CaF2�����¶Ⱥ�Ũ�ȵı仯���仯 | |
| B�� | ��1 L0.2 mol•L-1 HF��Һ�м���1 L 0.2 mol•L-1 CaCl2��Һ��û�г������� | |
| C�� | AgCl������ˮ������ת��ΪAgI | |
| D�� | ����AgCl����NaI��Һ�п�ʼת��ΪAgI��NaIŨ�ȱ��벻����$\frac{1}{\sqrt{1.8}}$��10-11 mol•L-1 |
��1������ͼIװ����ȡ��������ƿ�п�ѡ����Լ����������ƹ��壨��Ũ��ˮ���ʯ�һ�Ũ��ˮ����ʯ�ң��ȣ�
��2���Ʊ���������淋�װ������ͼ����ʾ����NH3��CO2ͨ�����Ȼ�̼�У����Ͻ����ϣ����ɵİ�������淋�С����������CCl4�У���������϶�ʱ��ֹͣ�Ʊ���

ע��CCl4��Һ��ʯ����Ϊ���Խ��ʣ�
��%2ͼI�еμ�Һ������������Ƿ�Һ©����Һ��ʯ������ƿ��������ͨ���۲����ݣ�����NH3��CO2ͨ���������ͨ���۲����ݣ�����NH3��CO2�ķ�Ӧ���ʣ����������ñ�ˮ��ȴ��ԭ���ǽ����¶ȣ���߷�Ӧ��ת���ʣ����¶ȣ���ֹ��Ӧ������ɲ���ֽ⣩��
�ڴӷ�Ӧ��Ļ�����з������Ʒ��ʵ�鷽���ǹ��ˣ���д�������ƣ���Ϊ�˵õ������Ʒ��Ӧ��ȡ�ķ�����b����дѡ����ţ���
a����ѹ���Ⱥ�� b����ѹ40�����º�� c����ѹ���Ⱥ��
��3���Ƶõİ�������刺��ܺ���̼����李�̼����е�һ�ֻ����֣�
����Ʒ��������гɷ�̽��������д���пո�
��ѡ�Լ�������ˮ��ϡHNO3��BaCl2��Һ��Ba��OH��2��Һ��AgNO3��Һ��ϡ���ᣮ
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ����������Ʒ���Թ��У���������ˮ�������ܽ⣮ | �õ���ɫ��Һ |
| ����2�����Թ��м��������BaCl2��Һ�����ã� | ��Һ����ǣ���֤�������к��У�NH4��2CO3�� |
| ����3��ȡ����2���ϲ���Һ���Թ��м���������Ba��OH��2��Һ�� | ��Һ������ǣ���֤�������в�����NH4HCO3�� |
| H2C2O4 | ��ɫ���� | K1=5.9��10-2��K2=6.4��10-5��������ˮ���Ҵ� |
| Na2C2O4 | ��ɫ���� | ����ˮ��pH=7.2���������Ҵ� |
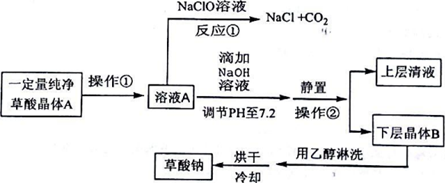
��ش��������⣺
��1��д����Ӧ�ٵĻ�ѧ����ʽH2C2O4+NaC1O=NaC1+2CO2��+H2O������������л�ԭ�ԣ�
��2������ҺA�м���NaOH��Һ����ʼ�μ��ٶ�Ҫ������Щ����Ŀ���������кͷ�Ӧ���ȣ����´ٽ���Ӧ��������Ӧ���ʣ����÷�Ӧ�ﵽ�յ�ʱ�Ļ�ѧ����ʽΪH2C2O4+2NaOH=Na2C2O4��+2H2O��
��3�������ڵ������ǹ��ˣ����Ҵ���ϴ����B��Ŀ���dz�ȥ�������ˮ�ּ�����ʧ
��4����0.01000mol/L�ĸ��������Һ�ζ�25.00mLijŨ�ȵIJ�������Һʱ����Ҫ����������ϡ���ᣬ��������ӦΪ��5C2O42-+2MnO4-+16H+�T2Mn2++10CO2��+8H2O�����������̫�࣬��������������ɲ�������Ͳ��ᣬʹ��Һ�в��������Ũ�Ƚ��ͣ�������Ӧ���ʣ�������������ʽ�ݶ��ܣ����ʽ����ʽ���������ﵽ��Ӧ�յ�ʱ����������Һ����dz��ɫ��30s�ڲ���ɫ����ô�ʱ�����������������Һ20.00mL����ò�������ҺŨ��Ϊ0.0200mol/L��
 ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£��ټ�ʽ�ζ���������ˮϴ�����ô�����Һ��ϴ����ע�������Һ�����ڵζ��ܵļ��첿�ֳ�����Һ����ʹҺ�洦�ڡ�0���̶����µ�λ�ã����¶���������ƿ������ˮϴ�����ô�����Һ��ϴ��ƿ2��3�Σ��Ӽ�ʽ�ζ����з���25.00mL������Һ����ƿ�У��ڽ���ʽ�ζ���������ˮϴ��������������ע��0.1000mol/L�����ᣬ���ڵζ��ܵļ��첿�ֳ�����Һ����ʹҺ�洦�ڡ�0���̶����µ�λ�ã����¶�����
ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£��ټ�ʽ�ζ���������ˮϴ�����ô�����Һ��ϴ����ע�������Һ�����ڵζ��ܵļ��첿�ֳ�����Һ����ʹҺ�洦�ڡ�0���̶����µ�λ�ã����¶���������ƿ������ˮϴ�����ô�����Һ��ϴ��ƿ2��3�Σ��Ӽ�ʽ�ζ����з���25.00mL������Һ����ƿ�У��ڽ���ʽ�ζ���������ˮϴ��������������ע��0.1000mol/L�����ᣬ���ڵζ��ܵļ��첿�ֳ�����Һ����ʹҺ�洦�ڡ�0���̶����µ�λ�ã����¶�����������ƿ�е����̪��ָʾ�������еζ����ζ���ָʾ��ǡ�ñ�ɫ���Ұ���Ӳ��仯�����������������ΪV1mL��
���ظ����Ϲ��̣����ζ�����������ƿ����5mL������ˮ�����������������ΪV2mL��
��1����ƿ�е���Һ�Ӻ�ɫ��Ϊ��ɫʱ��ֹͣ�ζ���
��2���ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧע����ƿ����Һ��ɫ�仯��
��3����С���ڲ�����еĴ�������ƿ�����ô���Һ��ϴ���ɴ���ɵIJⶨ���ƫ�ߣ���ƫ�ߡ�ƫ�ͻ���Ӱ�죩��
��4�������ȱ�ٵIJ������ñ�Һ��ϴ�ζ��ܣ�
��5����ͼ����ij�εζ�ʱ�ĵζ����е�Һ�棬�����Ϊ22.60mL��
��6�������������ݣ�
| �ζ����� | ����Һ�壨mL�� | �����������mL�� | |
| �ζ�ǰ����mL�� | �ζ��������mL�� | ||
| ��һ�� | 25.00 | 0.50 | 20.40 |
| �ڶ��� | 25.00 | 4.00 | 24.10 |

| A�� | ��װ�ü���ȡ���� | |
| B�� | ��װ��������FeBr3��Һ�е������� | |
| C�� | ��װ�ñ���Һʱ�ȴ��¿ڷų�ˮ�࣬�ٴ��Ͽڵ����л��� | |
| D�� | ��װ�ö�����Һ���ˮ���������ɣ��������Ƶ���ˮFeCl3 |
| A�� | AB2 | B�� | A2B | C�� | AB3 | D�� | A3B |
 ������ͭ�Ǻϳ��������������в���--��������ͭ����Ҫǰ��֮һ������������һ��ʵ���Һϳ�·�ߣ�
������ͭ�Ǻϳ��������������в���--��������ͭ����Ҫǰ��֮һ������������һ��ʵ���Һϳ�·�ߣ�

 ijѧ��Ϊ�ⶨδ֪Ũ�ȵ�������Һ����������ʵ�飺��1.00mL����������Һ����100mLϡH2SO4��Һ����0.14mol•L-1��NaOH��Һ�ζ�����ϡH2SO4 25.00mL���ζ���ֹʱ����NaOH��Һ15.00mL��
ijѧ��Ϊ�ⶨδ֪Ũ�ȵ�������Һ����������ʵ�飺��1.00mL����������Һ����100mLϡH2SO4��Һ����0.14mol•L-1��NaOH��Һ�ζ�����ϡH2SO4 25.00mL���ζ���ֹʱ����NaOH��Һ15.00mL��