��Ŀ����
8�������£������йص������Һ��˵����ȷ���ǣ�������| A�� | ����AgBr��AgI���������Һ��c��Ag+����c��Br-��=c��I-�� | |
| B�� | 25��ʱ��0.1mol•L-1������ҺPH=a��0.01mol•L-1������ҺPH=b����b=a+1 | |
| C�� | 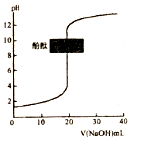 �����£�ͼ��ʾ�Է�̪��ָʾ������0.1mol•L-1NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ�ζ����յ�ʱ����Һһ�������� | |
| D�� | ��0.1mol•L-1�İ�ˮ�м�������粒��壬����Һ��c��OH-��/c��NH3•H2O����С |
���� A��AgBr��AgI�ܶȻ���ͬ��
B������Ϊ���ᣬŨ�Ȳ�ͬ�������̶Ȳ�ͬ��
C���ζ����յ�ʱ��pH����8-10֮�䣻
D����0.1mol•L-1�İ�ˮ�м�������粒��壬����һˮ�ϰ��ĵ��룮
��� �⣺A��AgBr��AgI�ܶȻ���ͬ��AgI�ܶȻ���С��ӦΪc��Br-����c��I-������A����
B������Ϊ���ᣬŨ��Խ�࣬����̶�ԽС��25��ʱ��0.1mol•L-1������ҺPH=a��0.01mol•L-1������ҺPH=b����b��a+1����B����
C���ζ����յ�ʱ��pH����8-10֮�䣬���Թ�������C����
D����0.1mol•L-1�İ�ˮ�м�������粒��壬����һˮ�ϰ��ĵ��룬��c��NH3•H2O������c��OH-����С������Һ��c��OH-��/c��NH3•H2O����С����D��ȷ��
��ѡD��
���� ���⿼���Ϊ�ۺϣ��漰���ܵ���ʵ��ܽ�ƽ�⣬������ʵĵ����Լ������ˮ���֪ʶ��Ϊ��Ƶ���㣬���ؿ���ѧ���ķ��������ͼ���������ע�����������ʵĵ����ص㣬�״���ΪB���Ѷ��еȣ�

��ϰ��ϵ�д�
 �ƸԹھ��ο���ϵ�д�
�ƸԹھ��ο���ϵ�д�
�����Ŀ
6�� ʵ�����Ʊ�1��2-������ķ�Ӧԭ�����£�
ʵ�����Ʊ�1��2-������ķ�Ӧԭ�����£�
CH3CH2OH$��_{170��}^{Ũ����}$CH2=CH2+H2O CH2=CH2+Br2��BrCH2CH2Br
���ܴ��ڵ���Ҫ����Ӧ�У��Ҵ���Ũ����Ĵ�������140����ˮ�������ѣ�������������������Ҵ��Ʊ�1��2-���������װ����ͼ��ʾ��
�����������
�ش��������⣺
��1���ڴ��Ʊ�ʵ���У�Ҫ������Ѹ�ٵذѷ�Ӧ�¶���ߵ�170�����ң�������ҪĿ����d������ȷѡ��ǰ����ĸ����
a��������Ӧ b���ӿ췴Ӧ�ٶ� c����ֹ�Ҵ��ӷ� d�����ٸ�������������
��2��װ��B�������ǰ�ȫƿ�����ã�
��3����װ��c��Ӧ����c������ȷѡ��ǰ����ĸ������Ŀ�������շ�Ӧ�п������ɵ��������壮
a��ˮ b��Ũ���� c������������Һ d������̼��������Һ
��4���жϸ��Ʊ���Ӧ����������������������ɫ��ȫ��ȥ��
��5����1��2-��������ֲ�Ʒ���ڷ�Һ©���м�ˮ�����ã�����Ӧ���²㣨��ϡ������¡�����
��6����������������δ��Ӧ��Br2�������b������ȷѡ��ǰ����ĸ��ϴ�ӳ�ȥ��
a��ˮ b������������Һ c���⻯����Һ d���Ҵ�
��7�������������������������ѣ���������ķ�����ȥ��
��8����Ӧ������Ӧ����ˮ��ȴװ��D������ҪĿ������ϩ���巴Ӧʱ���ȣ���ȴ�ɱ�����Ĵ����ӷ������ֲ�������ȴ�����ñ�ˮ������ԭ����1��2-������������̵�ϵͣ�9�棩��������ȴ��ʹ�����̶�ʹ��·������
 ʵ�����Ʊ�1��2-������ķ�Ӧԭ�����£�
ʵ�����Ʊ�1��2-������ķ�Ӧԭ�����£�CH3CH2OH$��_{170��}^{Ũ����}$CH2=CH2+H2O CH2=CH2+Br2��BrCH2CH2Br
���ܴ��ڵ���Ҫ����Ӧ�У��Ҵ���Ũ����Ĵ�������140����ˮ�������ѣ�������������������Ҵ��Ʊ�1��2-���������װ����ͼ��ʾ��
�����������
| �Ҵ� | 1��2-�������� | ���� | |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶȣ�g/cm3�� | 0.79 | 2.2 | 0.71 |
| �е㣨�棩 | 78.5 | 132 | 34.6 |
| �۵㣨�棩 | -130 | 9 | -116 |
��1���ڴ��Ʊ�ʵ���У�Ҫ������Ѹ�ٵذѷ�Ӧ�¶���ߵ�170�����ң�������ҪĿ����d������ȷѡ��ǰ����ĸ����
a��������Ӧ b���ӿ췴Ӧ�ٶ� c����ֹ�Ҵ��ӷ� d�����ٸ�������������
��2��װ��B�������ǰ�ȫƿ�����ã�
��3����װ��c��Ӧ����c������ȷѡ��ǰ����ĸ������Ŀ�������շ�Ӧ�п������ɵ��������壮
a��ˮ b��Ũ���� c������������Һ d������̼��������Һ
��4���жϸ��Ʊ���Ӧ����������������������ɫ��ȫ��ȥ��
��5����1��2-��������ֲ�Ʒ���ڷ�Һ©���м�ˮ�����ã�����Ӧ���²㣨��ϡ������¡�����
��6����������������δ��Ӧ��Br2�������b������ȷѡ��ǰ����ĸ��ϴ�ӳ�ȥ��
a��ˮ b������������Һ c���⻯����Һ d���Ҵ�
��7�������������������������ѣ���������ķ�����ȥ��
��8����Ӧ������Ӧ����ˮ��ȴװ��D������ҪĿ������ϩ���巴Ӧʱ���ȣ���ȴ�ɱ�����Ĵ����ӷ������ֲ�������ȴ�����ñ�ˮ������ԭ����1��2-������������̵�ϵͣ�9�棩��������ȴ��ʹ�����̶�ʹ��·������
3��NA��������٤��������ֵ������������ȷ���ǣ�������
| A�� | �����£���1LpH=1�Ĵ�����Һ�м�ˮ��������Һ��H+��Ŀ����0.1NA | |
| B�� | 60g�����������Ҵ�����������Ӧ����ַ�Ӧ����ѵ�C-O����ĿΪNA | |
| C�� | ��ˮ�Ҵ������������Ʒ�Ӧ����5.6LH2�����Ҵ������й��ۼ�����Ϊ4NA | |
| D�� | ��֪C2H4��g��+H2��g���TC2H6��g����H=-137.0kJ/mol����ϩ��H2�ӳ�ʱ�ų�137.0kJ���� |
13������ʵ���У���Ӧ�������Լ����۶���ȷ�����߾��������ϵ���ǣ�������
| ѡ�� | ʵ�� | ���� | ���� |
| A | ��KI��Һ�м���CCl4������ | Һ��ֲ㣬�²���Ϻ�ɫ | ��������CCl4��������ˮ |
| B | ������ǯ��סһС����ɰֽ��ϸ��ĥ���������ھƾ����ϼ��� | �ۻ����Һ̬���������� | ���������۵�ϵ� |
| C | ���з�̪��Na2CO3��Һ�м���������BaCl2���� | ��Һ��ɫ��dz | ֤��Na2CO3��Һ�д���ˮ��ƽ�� |
| D | ���������������е��������ữ��Ba��ClO��2��Һ | ������ɫ���� | �������������� |
| A�� | A | B�� | B | C�� | C | D�� | D |
20����ѧ��������������������أ������й�˵����ȷ���ǣ�������
| A�� | ��ȼ�������ȼ������SO3 | |
| B�� | �й��Ŵ�����������Һ���������ͭ��������ͭ�� | |
| C�� | ���ð�˾ƥ�ֳ���ˮ���ᷴӦʱ����NaOH��Һ�ⶾ | |
| D�� | ʹ�ú�������Ũ�Ƚϴ�ĵ���ˮϴ�·�������ȥ��������ǿ |
17��������ȫ���ҵ���ʱ�ķ�ӦΪ��10NaN3+2KNO3+6SiO2$\frac{\underline{\;ײ��\;}}{\;}$5Na2SiO3+K2SiO3+16N2��������˵����ȷ���ǣ�������
| A�� | ��Ȼ���е����ơ���Ԫ�ؾ���������̬��ʽ���� | |
| B�� | ����Ӧ��Ĺ�����������ˮ�����ã�ͨ��CO2�������� | |
| C�� | ���İ뾶��r��K+����r��Na+����r��O2-�� | |
| D�� | ����Ӧ��ת��6.02��1022���ӣ�������NaN3������Ϊ6.5g |
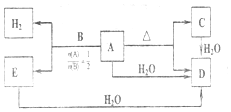
 ��
�� ��C��D��Ӧ���ɵĻ�����ĵ���ʽ��
��C��D��Ӧ���ɵĻ�����ĵ���ʽ�� ��
�� ���ݻ�Ϊ2L���ܱ������У�Ͷ��2molA��3molB������Ӧ��A��s��+2B��g��?2C��g����B�����ʵ����ı仯��ͼ��ʾ����֪��2��t1ʱ�ε�����Ӧ���ʱ�t2��t3ʱ�ε�����Ӧ���ʿ죮
���ݻ�Ϊ2L���ܱ������У�Ͷ��2molA��3molB������Ӧ��A��s��+2B��g��?2C��g����B�����ʵ����ı仯��ͼ��ʾ����֪��2��t1ʱ�ε�����Ӧ���ʱ�t2��t3ʱ�ε�����Ӧ���ʿ죮