��Ŀ����
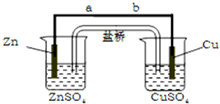
5��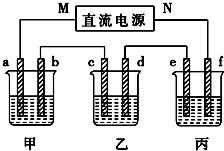 ��ͼ��ʾװ���У��ס��ҡ��������ձ����ηֱ�ʢ��100g 5.00%��NaOH��Һ��������CuSO4��Һ��100g 10.00%��K2SO4��Һ���缫��Ϊʯī�缫����ͨ��Դ������һ��ʱ���ñ���K2SO4Ũ��Ϊ10.47%������c�缫�������ӣ��ݴ˻ش����⣺
��ͼ��ʾװ���У��ס��ҡ��������ձ����ηֱ�ʢ��100g 5.00%��NaOH��Һ��������CuSO4��Һ��100g 10.00%��K2SO4��Һ���缫��Ϊʯī�缫����ͨ��Դ������һ��ʱ���ñ���K2SO4Ũ��Ϊ10.47%������c�缫�������ӣ��ݴ˻ش����⣺�ٵ�Դ��N��Ϊ������
�ڵ缫b�Ϸ����ĵ缫��ӦΪ4OH--4e-=2H2O+O2����
�۵��ǰ�����Һ��pH�Ƿ����仯��������ԭ��
����Һ����
����Һ��С��
����Һ���䣻
����ʽ����缫a�����ɵ������ڱ�״���µ����Ϊ2.8L��
�ݵ缫c�������仯��16g��
���� ������c�缫�������ӣ���d�������ķ�ӦΪ��Cu2++2e-=Cu����d��Ϊ�������ɴ˿��Ƴ�cΪ�������Դ˿�ȷ�������缫�͵�Դ����������
�ڼ���ΪNaOH���൱�ڵ��H2O������b��Ϊ������OH-�ŵ磻
�ۼ����൱�ڵ��ˮ����NaOH��Ũ������pH�����������ΪCu2+�ŵ磬����ΪOH-�ŵ磬����H+���࣬��pH��С������Ϊ���ˮ������K2SO4���ԣ���pH�������䣻
�������ˮ������Ϊx���ɵ��ǰ��������������У�100��10%=��100-x����10.47%����x=4.5g����Ϊ0.25mol���ɷ���ʽ2H2+O2�T2H2O��֪������2molH2O��������Ӧ��ת��0.5mol���ӣ�a��������������ϵ���ת�ƺ͵缫����ʽ���������
��Cu2++2e-=Cu��ת��0.5mol���ӣ��Դ˿ɼ��������仯��
��� �⣺���ұ���c�������ӣ�˵��Cu������c�缫�ϣ������Ǵ�b-c�ƶ���M�Ǹ�����NΪ�������ʴ�Ϊ������
�ڼ���ΪNaOH���൱�ڵ��H2O������b��Ϊ������OH-�ŵ磬��4OH--4e-=2H2O+O2�����ʴ�Ϊ��4OH--4e-=2H2O+O2����
�ۼ����൱�ڵ��ˮ����NaOH��Ũ������pH�����������ΪCu2+�ŵ磬����ΪOH-�ŵ磬��ⷽ��ʽΪ��2CuSO4+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2Cu+O2��+2H2SO4������H+���࣬��pH��С������Ϊ���ˮ������K2SO4���ԣ���pH�������䣬
�ʴ�Ϊ������С�����䣻
�ܱ���ΪK2SO4���൱�ڵ��ˮ�������ˮ������Ϊx���ɵ��ǰ��������������У�100��10%=��100-x����10.47%����x=4.5g����Ϊ0.25mol��
�ɷ���ʽ2H2+O2�T2H2O��֪������2molH2O��ת��4mol���ӣ�����������Ӧ��ת��0.5mol���ӣ�
�缫a������4OH--4e-=2H2O+O2����
������O2Ϊ$\frac{0.5mol}{4}$=0.125mol������µ����Ϊ0.125��22.4=2.8L��
�ʴ�Ϊ��2.8L��
��������·�Ǵ����ģ�����ÿ���ձ��еĵ缫��ת�Ƶ���������ȵģ����ݵ缫��Ӧ��Cu2++2e-=Cu����֪ת��0.5mol�������ɵ�m��Cu��=$\frac{0.5}{2}$mol��64g/mol=16g��
�ʴ�Ϊ��16g��
���� ����Ϊ�绯ѧ֪ʶ���ۺ�Ӧ�ã�����ʱҪע����ݵ缫��Ӧ�����жϳ����ص��������������жϳ���Դ����������Ҫע����������Ϊ������·�����缫�ϵ�ʧ���ӵ���Ŀ��ȣ�����ʱҪ��ȷд���缫����ʽ��ȷ�ж����������ӵķŵ�˳��

 ��У����ϵ�д�
��У����ϵ�д�| A�� | ��������������һֱ���ֺ��� | |
| B�� | ����Ϊ������Ag2S���������ɵ����� | |
| C�� | �ù������ܷ�ӦΪ2Al+3Ag2S�T6Ag+Al2S3 | |
| D�� | ��ɫ��ȥ��ԭ���Ǻ�ɫAg2Sת��Ϊ��ɫAg |
| A�� | ����ͭ��Һ����������������Һ��ϣ�Ba2++SO42-�TBaSO4�� | |
| B�� | �ô����ˮ����2CH3COOH+CaCO3�T2CH3COO-+Ca2++H2O+CO2�� | |
| C�� | ���Ƶ�ˮ�ⷴӦ��S2-+H3O+?HS-+H2O | |
| D�� | SO2ͨ���ˮ�У���Ӧ�����ӷ���ʽΪ��SO2+I2+2H2O�TSO32-+2I-+4H+ |
| A�� | ˫��ˮ����Ϊ��ɫ������������Ϊ�仹ԭ����ͨ��ΪO2���Ի���û����Ⱦ | |
| B�� | ��ˮ��Ũ�����Ũ���ᰴ�����3��1��ɵĻ��������ܽ�Au��Pt | |
| C�� | ��ˮ�������ԣ�Ũ��Խ���ܶ�Խ�� | |
| D�� | ���Ʊ�����ˮ�д������ַ��ӣ��������� |
| A�� | ������ O2 �ݳ� | |
| B�� | ͭƬ���� H2 �ݳ� | |
| C�� | ����ͨ��������ͭƬ����пƬ | |
| D�� | ���������� SO42- ����Ũ�������� |
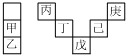 �ס���������Ԫ�������ڱ��е����λ�����±�����������������Ӧ��ˮ������ǿ��ˮ�ԣ��Ͷ���ͬһ���ڣ���ԭ������������ڲ������ͬ�������������ж���ȷ���ǣ�������
�ס���������Ԫ�������ڱ��е����λ�����±�����������������Ӧ��ˮ������ǿ��ˮ�ԣ��Ͷ���ͬһ���ڣ���ԭ������������ڲ������ͬ�������������ж���ȷ���ǣ�������| A�� | ��̬�⻯����ȶ��ԣ����������� | |
| B�� | �������ԭ���������24 | |
| C�� | ������γɵĵ������Ӱ뾶��СΪ�������� | |
| D�� | ����������������������������оƬ |