��Ŀ����
���ڳ��о���ʹ�õĹܵ�ú������Ҫ�ɷ���H2��CO������CH4��H2��CO��CH4��ȼ�����������±���
��1����д��H2��CO��CH4ȼ�յ��Ȼ�ѧ����ʽ�� �� �� ��
��2����Ϊ�����������������乤�̵���Ҫ�ɾͣ�������Ȼ����ȫ������Ϻ������վ��ڣ�ʹ�ùܵ�ú���û�������Ȼ����Ӧ������߽��������ţ������� �������������Ȼ�������Ľ���������� �������������Ȼ�������Ľ��������ݱ�����ͬʱȼ���۸�Ҳ�������е�0.95Ԫ/m3�Ļ����ϵ�����1.31Ԫ/m3����ͨ������˵������������ˮƽ���䣬������ȼ����������Ѵ�Լ�����ڵ� ��������ȷ��С�����2λ��
| ���� | H2 | CO | CH4 |
| ȼ����kJ?mol-1 | 285.8 | 283.0 | 890.3 |
��2����Ϊ�����������������乤�̵���Ҫ�ɾͣ�������Ȼ����ȫ������Ϻ������վ��ڣ�ʹ�ùܵ�ú���û�������Ȼ����Ӧ������߽��������ţ�������
���㣺�Ȼ�ѧ����ʽ,��ѧ��Ӧ���ʵ�Ӱ������
ר�⣺
��������1��ȼ������ָ1mol��ȼ����ȫȼ�������ȶ�������ų������������ݸ���д���Ȼ�ѧ����ʽ��
��2�����ݻ�ѧ����ʽ�е������Ӹ����Ƚ��з������������Ķ��٣�����ȼ��ͬ����������Ȼ����һ����̼����Ȼ��ȼ����Ҫ�������࣬ȼ�չܵ�ú����������������Ȼ������Ҫ�Ӵ�����������������Ҫ��ͬ���������Դ˼��㣮
��2�����ݻ�ѧ����ʽ�е������Ӹ����Ƚ��з������������Ķ��٣�����ȼ��ͬ����������Ȼ����һ����̼����Ȼ��ȼ����Ҫ�������࣬ȼ�չܵ�ú����������������Ȼ������Ҫ�Ӵ�����������������Ҫ��ͬ���������Դ˼��㣮
���
�⣺��1��ȼ������ָ1mol��ȼ����ȫȼ�������ȶ�������ų�������������H2��CO��CH4ȼ�յ��Ȼ�ѧ����ʽΪ��
H2��g��+
O2��g��=H2O��l����H=-285.8KJ/mol��
CO��g��+
O2=CO2��g����H=-283.0KJ/mol��
CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-890.3KJ/mol
�ʴ�Ϊ��H2��g��+
O2��g��=H2O��l����H=-285.8KJ/mol��CO��g��+
O2=CO2��g����H=-283.0KJ/mol��CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-890.3KJ/mol��
��2���ɻ�ѧ����ʽ�������Ӹ����ȿ�֪��ȼ����ͬ����Ĺܵ�ú������Ȼ��ʱ����Ȼ�����ĵ������ࣻȼ�չܵ�ú������������ȼ��Ȼ������ߵĸĽ��������������ڣ����ȼ�ϲ���ȫȼ�գ�������һ����̼����Ⱦ���������ʣ�����ʹ�ùܵ�ú���û�������Ȼ����Ӧ������߽��������ţ�����������Ľ������������Ȼ���Ľ��������������������ˮƽ��Ҫ����ΪXKJ����ʹ��ˮú����Ҫ
��
��0.95=
��
��������Ȼ����Ҫ��
��1.31����������ȼ����������Ѵ�Լ�����ڵ�
=0.44��
�ʴ�Ϊ����������Ȼ����0.44��
H2��g��+
| 1 |
| 2 |
CO��g��+
| 1 |
| 2 |
CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-890.3KJ/mol
�ʴ�Ϊ��H2��g��+
| 1 |
| 2 |
| 1 |
| 2 |
��2���ɻ�ѧ����ʽ�������Ӹ����ȿ�֪��ȼ����ͬ����Ĺܵ�ú������Ȼ��ʱ����Ȼ�����ĵ������ࣻȼ�չܵ�ú������������ȼ��Ȼ������ߵĸĽ��������������ڣ����ȼ�ϲ���ȫȼ�գ�������һ����̼����Ⱦ���������ʣ�����ʹ�ùܵ�ú���û�������Ȼ����Ӧ������߽��������ţ�����������Ľ������������Ȼ���Ľ��������������������ˮƽ��Ҫ����ΪXKJ����ʹ��ˮú����Ҫ
| X |
| 285.8+283.0 |
| 1 |
| 2 |
| 0.95X |
| 568.8 |
| 1 |
| 2 |
| X |
| 890.3 |
| ||
|
�ʴ�Ϊ����������Ȼ����0.44��
���������⿼�����Ȼ�ѧ����ʽ����д��ȼ���ȵĸ���Ӧ�ã��Ȼ�ѧ����ʽ�ĵ��������жϣ�ע�����һ�ʼ���Ƚϸ��ӣ�ץס����ˮƽ����ͽϼ�

��ϰ��ϵ�д�
�����Ŀ
����˵����ȷ���ǣ�������
| A������Ԫ����ԭ�Ӱ뾶��С����F |
B�����ݦм��ijɼ������ж�C-C�ļ�����  ���ܵĹ�ϵ��˫���ļ���С�ڵ����ļ��ܵ�2�� ���ܵĹ�ϵ��˫���ļ���С�ڵ����ļ��ܵ�2�� |
| C��Ԫ�ص縺��ԽС��Ԫ�طǽ�����Խǿ |
| D����n���ڵ�n�����Ԫ�ؾ�Ϊ���� |
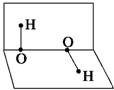 ��֪H2O2�ķ��ӿռ�ṹ���ڶ�����б�ʾ����ͼ��ʾ�����й�H2O2�Ľṹ��˵���в���ȷ���ǣ�������
��֪H2O2�ķ��ӿռ�ṹ���ڶ�����б�ʾ����ͼ��ʾ�����й�H2O2�Ľṹ��˵���в���ȷ���ǣ�������| A�����ӵ�������������IJ��غ� |
| B��H2O2�����ڼȺ����Լ��ֺ��Ǽ��Լ� |
| C��H2O2�Ǽ��Է��� |
| D��H2O2���Ӽ䲻�����γ���� |
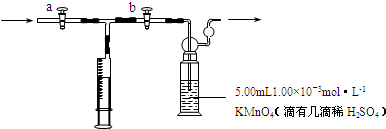

 A����;���Ľ�����B��C�����ֳ������嵥�ʣ�E��ҺΪ����ǿ�ᣬD��Һ�еμ�KSCN��Һ�Ժ�ɫ�������ת����ϵ��ͼ��ʾ��
A����;���Ľ�����B��C�����ֳ������嵥�ʣ�E��ҺΪ����ǿ�ᣬD��Һ�еμ�KSCN��Һ�Ժ�ɫ�������ת����ϵ��ͼ��ʾ��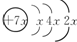 ��B��ͬ���ڳ�ϡ��������뾶����Ԫ�أ�C������������������ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
��B��ͬ���ڳ�ϡ��������뾶����Ԫ�أ�C������������������ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺