��Ŀ����
�������ᴿ��������ʵij��÷����У��ٹ��ˡ��ڽᾧ������������������ȡ�������������ȷֽ���Һ�ȣ���������ᴿ�ı���������и���������ĺ����ϣ�
��1����ȥ̼���ƹ����л��еĵⵥ�� ��
��2����ȥFe��OH��3�����е�FeCl3 ��
��3��������غ������Ȼ�����ɵĹ�������ᴿ����� ��
��4����ȥ����ֲ�����е�ˮ ����
��5�����յ��CCl4��Һ�е�CCl4 ��
��6����ʳ�þƾ������в�ҩ��ȡ���е���Ч�ɷ� ��
��1����ȥ̼���ƹ����л��еĵⵥ��
��2����ȥFe��OH��3�����е�FeCl3
��3��������غ������Ȼ�����ɵĹ�������ᴿ�����
��4����ȥ����ֲ�����е�ˮ
��5�����յ��CCl4��Һ�е�CCl4
��6����ʳ�þƾ������в�ҩ��ȡ���е���Ч�ɷ�
���㣺���ʵķ��롢�ᴿ�Ļ�������ѡ����Ӧ��
ר�⣺��ѧʵ���������
��������1������������
��2�����岻������Ĥ��
��3������غ��Ȼ��ص��ܽ�Ȳ�ͬ��
��4��ֲ���Ͳ�����ˮ��
��5��������Ȼ�̼�ķе㲻ͬ��
��6���л�ҩƷ�ھƾ��е��ܽ�Ƚϴ�
��2�����岻������Ĥ��
��3������غ��Ȼ��ص��ܽ�Ȳ�ͬ��
��4��ֲ���Ͳ�����ˮ��
��5��������Ȼ�̼�ķе㲻ͬ��
��6���л�ҩƷ�ھƾ��е��ܽ�Ƚϴ�
���
�⣨1���������������������ķ������룬�ʴ�Ϊ���ۣ�
��2�����岻������Ĥ�����������ķ������룬�ʴ�Ϊ���ޣ�
��3������غ��Ȼ��ص��ܽ�Ȳ�ͬ�����ýᾧ�����룬�ʴ�Ϊ���ڣ�
��4��ˮ��ֲ���ͻ������ܣ����÷�Һ�ķ������룬�ʴ�Ϊ���ࣻ
��5��������Ȼ�̼�ķе㲻ͬ����������ķ������룬�ʴ�Ϊ���ܣ�
��6���л�ҩƷ�ھƾ��е��ܽ�Ƚϴ�Ϊ��ȡ�������ʴ�Ϊ���ݣ�
��2�����岻������Ĥ�����������ķ������룬�ʴ�Ϊ���ޣ�
��3������غ��Ȼ��ص��ܽ�Ȳ�ͬ�����ýᾧ�����룬�ʴ�Ϊ���ڣ�
��4��ˮ��ֲ���ͻ������ܣ����÷�Һ�ķ������룬�ʴ�Ϊ���ࣻ
��5��������Ȼ�̼�ķе㲻ͬ����������ķ������룬�ʴ�Ϊ���ܣ�
��6���л�ҩƷ�ھƾ��е��ܽ�Ƚϴ�Ϊ��ȡ�������ʴ�Ϊ���ݣ�
���������⿼�����ʵķ��롢�ᴿ������Ϊ��Ƶ���㣬������ѧ���ķ���������ʵ�������Ŀ��飬��Ŀ�ѶȲ�����ȷ���ʵ����ʵ���ͬ�ǽ�������Ŀ�Ĺؼ���ע����ػ���֪ʶ�Ļ��ۣ�

��ϰ��ϵ�д�
�����Ŀ
������ʵ����ص�������ȷ���ǣ�������
| A���ⶨ��ҺpHʱ������pH��ֽպȡ����Һ���������ɫ������ |
| B����ȼ�Լ���ǿ�������Լ��ֿ����ò�Զ���Դ |
| C������ij��Һ�Ƿ���SO42-ʱ��Ӧȡ��������Һ�����μ����Ȼ�����Һ��ϡ���� |
| D��ϴ�ӳ���ʱ����©���м�����ˮ�����貢�˸� |
��ɫ��ѧ�ĺ����Ƿ�Ӧ���̵���ɫ������Ҫ��ԭ�������е�����ԭ����ȫ��������ȫ��ת�������IJ�Ʒ�У����й��̲�������һ˼����ǣ�������
A�����顢CO�ϳ���������2CH4+2CO
| ||
B��ϩ����ˮú���������ʻ��ϳɷ�ӦRCH=CH2+CO+H2
| ||
C��������������Ӧ���ȷ£�CH4+3Cl2
| ||
| D����ϩ�ϳɾ���ϩ |
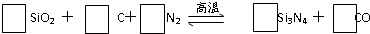
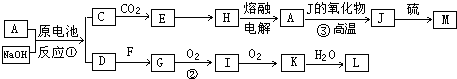 A��J���ճ������г��������ֽ����������ֽ�����NaOH���ԭ��أ�A��������F�����������嵥�ʣ������������µ�ת����ϵ�����ֲ��P������ȥ����
A��J���ճ������г��������ֽ����������ֽ�����NaOH���ԭ��أ�A��������F�����������嵥�ʣ������������µ�ת����ϵ�����ֲ��P������ȥ����