��Ŀ����
11��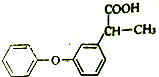 �밴Ҫ��ش��������⣺
�밴Ҫ��ش��������⣺��1����ŵ������������ʪ�Թؽ���ҩ���ṹ��ʽ��ͼ��
�ٷ�ŵ��ҵĺ�������������Ϊ�Ȼ����ѻ����ڷ�ŵ����ܷ������л���Ӧ�����мӳɡ�ȡ����Ӧ��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ��
���ڼ��ȡ���ѹ�ʹ�����������ϩ��ˮ��Ӧ��CH2=CH2+H2O$��_{��}^{����}$CH3CH2OH��
��CH2=CHCOOCH2CH3�ľۺϷ�Ӧ��nCH2=CHCOOCH2CH3$\stackrel{����}{��}$
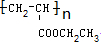 ��
��
���� ��1�� ��-COOH��-C-O-C���ɷ���ȡ�����ӳɷ�Ӧ��
��-COOH��-C-O-C���ɷ���ȡ�����ӳɷ�Ӧ��
��2������ϩ����̼̼˫��������ˮ�����ӳɷ�Ӧ�����Ҵ���
��CH2=CHCOOCH2CH3����̼̼˫�����ɷ����Ӿ۷�Ӧ��
��� �⣺��1���л��ﺬ�еĹ�����Ϊ�Ȼ����ѻ����Һ��б������ɷ����ӳɡ�ȡ����������Ӧ�����ʴ�Ϊ���Ȼ����ѻ����ӳɡ�ȡ����Ӧ��
��2����CH2=CH2�в����͵�C=C˫��������1��C-C���ѽ��ˮ�ṩ��-H��-OH�������Ҵ�������ʽΪ��CH2=CH2+H2O$��_{��}^{����}$ CH3CH2OH��
�ʴ�Ϊ��CH2=CH2+H2O$��_{��}^{����}$ CH3CH2OH��
��CH2=CHCOOCH2CH3�ľۺϷ�ӦΪnCH2=CHCOOCH2CH3$\stackrel{����}{��}$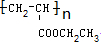 ���ʴ�Ϊ��nCH2=CHCOOCH2CH3$\stackrel{����}{��}$
���ʴ�Ϊ��nCH2=CHCOOCH2CH3$\stackrel{����}{��}$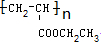 ��
��
���� ���⿼���л���Ľṹ�����ʣ�Ϊ��Ƶ���㣬������ѧ���ķ��������Ŀ��飬ע������л���Ľṹ�����ŵ����ʣ������л���������������ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
2������ʵ�������ʵ���¹ʴ�����ȷ���ǣ�������
| A�� | ������Ũ����Һմ��Ƥ���ϣ������ô��������ϴ��Ȼ������ˮ��ϴ | |
| B�� | �������ὦ�����У������ô���ˮ��ϴ����ϴ��գ�۾� | |
| C�� | ʵ��ʱ��ָ�ϲ�С��մ��Ũ���ᣬ������NaOH��Һ��ϴ | |
| D�� | �ƾ���������ʵ������������ʱ��������ˮ���� |
19������ָ����Ӧ�����ӷ���ʽ��ȷ���ǣ�������
| A�� | ��������ˮ��Cl2+H2O�T2H++Cl-+ClO- | |
| B�� | ��CuSO4��Һ�м���NaOH��Cu2++2OH-�TCu��OH��2�� | |
| C�� | ��������Һ�У�KIO3��KI��Ӧ����I2��IO3-+I-+6H+�TI2+3H2O | |
| D�� | ��Al2��SO4��3��Һ�м��������NH3?H2O2��Al3++4NH3?H2O2�TAlO2-+4NH4++2H2O |
6��ij���᳧��������������Ҫ��Fe2O3��FeO��������һ������SiO2���������Ʊ�FeCO3����������ͼ��
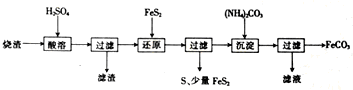
��֪����ԭʱ��FeS2��H2SO4����Ӧ��Fe3+ͨ����Ӧ��ԭ�����з�Ӧ��ΪFeS2+14Fe3++8H2O�T15Fe2++2SO42-+16H+��
��1����Ӧ���л�ԭ����FeS2��
��2�������ijɷ��Ƕ������裨�����ƣ���
��3������FeCO3����ϴ�ӣ�����FeCO3�Ƿ�ϴ���ķ�����ȡ�������һ��ϴ��Һ���Թ��У��μ�ϡ���ᣬ��������������ٵμ�BaCl2��Һ�����ް�ɫ�������ɣ��������ϴ�Ӹɾ�����֮����˵��ûϴ�Ӹɾ���
��4������ƽ��Ӧ������ӷ���ʽ��2Fe3++1FeS2=2S��+3Fe2+��
�ڻ�ԭǰ����Һ�в������ӵ�Ũ�ȼ�������Һ����仯���Բ��ƣ���
��Ӧ��������Fe2+�����ʵ���֮��Ϊ5��3��

��֪����ԭʱ��FeS2��H2SO4����Ӧ��Fe3+ͨ����Ӧ��ԭ�����з�Ӧ��ΪFeS2+14Fe3++8H2O�T15Fe2++2SO42-+16H+��
��1����Ӧ���л�ԭ����FeS2��
��2�������ijɷ��Ƕ������裨�����ƣ���
��3������FeCO3����ϴ�ӣ�����FeCO3�Ƿ�ϴ���ķ�����ȡ�������һ��ϴ��Һ���Թ��У��μ�ϡ���ᣬ��������������ٵμ�BaCl2��Һ�����ް�ɫ�������ɣ��������ϴ�Ӹɾ�����֮����˵��ûϴ�Ӹɾ���
��4������ƽ��Ӧ������ӷ���ʽ��2Fe3++1FeS2=2S��+3Fe2+��
�ڻ�ԭǰ����Һ�в������ӵ�Ũ�ȼ�������Һ����仯���Բ��ƣ���
| ���� | ����Ũ�ȣ�mol?L-1�� | |
| ��ԭǰ | ��ԭ�� | |
| Fe2+ | 0.10 | 2.50 |
| SO42- | 3.50 | 3.70 |
16����0.2molCO2ͨ��װ������Na2O2�ĸ�����У���������ص�����Ϊ��������
| A�� | 2.8g | B�� | 5.6g | C�� | 8.8g | D�� | 11.2g |
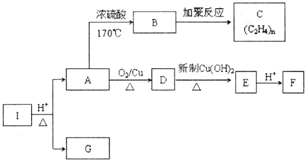 ������I�ķ���ʽΪC6H10O4��75%��A��Һ������ҽ����������I��صķ�Ӧ��ͼ������������Ϣ�ش��������⣮
������I�ķ���ʽΪC6H10O4��75%��A��Һ������ҽ����������I��صķ�Ӧ��ͼ������������Ϣ�ش��������⣮ ��
��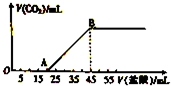 ��5.08g��Na2CO3��NaHCO3��ɵĹ���������ȫ����ˮ���Ƴ���Һ��Ȼ�������Һ����μ���2mol?L-1�����ᣬ�������������������CO2�������״�����Ĺ�ϵ��ͼ��ʾ��
��5.08g��Na2CO3��NaHCO3��ɵĹ���������ȫ����ˮ���Ƴ���Һ��Ȼ�������Һ����μ���2mol?L-1�����ᣬ�������������������CO2�������״�����Ĺ�ϵ��ͼ��ʾ�� ijͬѧ��װ����ͼ��ʾ�ĵ绯ѧװ�ã��缫��ΪAl�������缫��ΪCu��
ijͬѧ��װ����ͼ��ʾ�ĵ绯ѧװ�ã��缫��ΪAl�������缫��ΪCu�� ������Ϊ
������Ϊ ���䵥��Ľṹ��ʽ�ֱ�Ϊ
���䵥��Ľṹ��ʽ�ֱ�Ϊ ��
�� ��
��