��Ŀ����
��֪�л���A��C���dz����еij��õ���Ʒ��A��һ����ɫ����������ζ��Һ�壬��Է���������������23����A��Cu���£��������ڼ����������������л���B���л���C��һ����ǿ�Ҵ̼�����ζ����ɫҺ�壬��ʹ��ɫʯ����Һ��죬����A��C��������һ���з�����ζ��Һ�壮ijͬѧ�������ʵ�飬�������ĿҪ����д���пո�
��1���������ƶ�A�Ľṹ��ʽΪ ����ͼ1��ʾ���Թ���װA������������Ϊ ���ѧʽ����
��2����ͼ2��ʾ���Ѽ��ȵ�ͭ˿���뵽װ��A���Թ��У��ŵ��д̼�����ζ���÷�Ӧ�в������л���ΪB��B���ܷ���������Ӧ����B������Ϊ ���ṹ��ʽΪ�� ��
��3��C��ʹ��ɫʯ����Һ�� ɫ��˵��C���� �ԣ��ᡢ���C�Ľṹ��ʽΪ
��4����C������ˮ������Ҫ�ɷ�CaCO3����ˮ�����������ˮ��˵��C������ ̼������ԣ����ǿ�ڡ������ڡ���
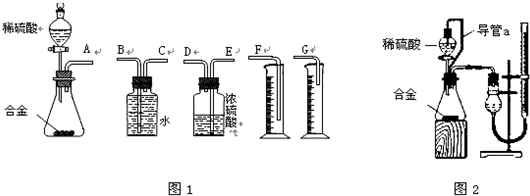
��5������ͼ3��ʾװ�ã�A��C�ܷ���������Ӧ����д���÷�Ӧ�Ļ�ѧ����ʽ�� ��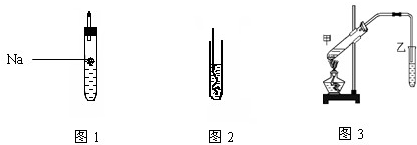
��1���������ƶ�A�Ľṹ��ʽΪ
��2����ͼ2��ʾ���Ѽ��ȵ�ͭ˿���뵽װ��A���Թ��У��ŵ��д̼�����ζ���÷�Ӧ�в������л���ΪB��B���ܷ���������Ӧ����B������Ϊ
��3��C��ʹ��ɫʯ����Һ��
��4����C������ˮ������Ҫ�ɷ�CaCO3����ˮ�����������ˮ��˵��C������
��5������ͼ3��ʾװ�ã�A��C�ܷ���������Ӧ����д���÷�Ӧ�Ļ�ѧ����ʽ��

���㣺�л�����ƶ�
ר�⣺�л���Ļ�ѧ���ʼ��ƶ�
�������л���A��C���dz����еij��õ���Ʒ��A��һ����ɫ����������ζ��Һ�壬��Է���������������23������Mr��A��=23��2=46��A��Cu���£��������ڼ����������������л���B����AΪCH3CH2OH��BΪCH3CHO���л���C��һ����ǿ�Ҵ̼�����ζ����ɫҺ�壬��ʹ��ɫʯ����Һ��죬����-COOH������A��C��������һ���з�����ζ��Һ�壬��CΪCH3COOH���ݴ˽��
���
�⣺�л���A��C���dz����еij��õ���Ʒ��A��һ����ɫ����������ζ��Һ�壬��Է���������������23������Mr��A��=23��2=46��A��Cu���£��������ڼ����������������л���B����AΪCH3CH2OH��BΪCH3CHO���л���C��һ����ǿ�Ҵ̼�����ζ����ɫҺ�壬��ʹ��ɫʯ����Һ��죬����-COOH������A��C��������һ���з�����ζ��Һ�壬��CΪCH3COOH��
��1��������������֪��A�Ľṹ��ʽΪCH3CH2OH�������Ҵ���Ӧ�����Ҵ�����������ͼ1װ���в���������ΪH2���ʴ�Ϊ��CH3CH2OH��H2��
��2���Ҵ���Cu���£��������ڼ����������������л���BΪ��ȩ���ṹ��ʽΪCH3CHO���ʴ�Ϊ����ȩ��CH3CHO��
��3��CΪ���ᣬ�������ͨ�ԣ���ʹ��ɫʯ����Һ���ɫ��C�Ľṹ��ʽΪCH3COOH���ʴ�Ϊ���죻�CH3COOH��
��4����C������ˮ������Ҫ�ɷ�CaCO3����ˮ�����������ˮ��˵��C������ǿ��̼������ԣ��ʴ�Ϊ��ǿ�ڣ�
��5���������Ҵ�����������Ӧ���������������÷�Ӧ�Ļ�ѧ����ʽΪ��CH3COOH+CH3CH2OH
CH3COOCH2CH3+H2O���ʴ�Ϊ��CH3COOH+CH3CH2OH
CH3COOCH2CH3+H2O��
��1��������������֪��A�Ľṹ��ʽΪCH3CH2OH�������Ҵ���Ӧ�����Ҵ�����������ͼ1װ���в���������ΪH2���ʴ�Ϊ��CH3CH2OH��H2��
��2���Ҵ���Cu���£��������ڼ����������������л���BΪ��ȩ���ṹ��ʽΪCH3CHO���ʴ�Ϊ����ȩ��CH3CHO��
��3��CΪ���ᣬ�������ͨ�ԣ���ʹ��ɫʯ����Һ���ɫ��C�Ľṹ��ʽΪCH3COOH���ʴ�Ϊ���죻�CH3COOH��
��4����C������ˮ������Ҫ�ɷ�CaCO3����ˮ�����������ˮ��˵��C������ǿ��̼������ԣ��ʴ�Ϊ��ǿ�ڣ�
��5���������Ҵ�����������Ӧ���������������÷�Ӧ�Ļ�ѧ����ʽΪ��CH3COOH+CH3CH2OH
| Ũ���� |
| �� |
| Ũ���� |
| �� |
���������⿼���л����ƶϣ��漰����ȩ�������������ת�����Ƚϻ��������ضԻ���֪ʶ�Ĺ��̣�

��ϰ��ϵ�д�
�����Ŀ
�й�������ԭ��Ӧ��������ȷ���ǣ�������
| A��������ԭ��Ӧ��Ȼ����Ԫ�صĵ�ʧ |
| B���û���Ӧһ������������ԭ��Ӧ |
| C��ijԪ���ڻ�ѧ��Ӧ���ɻ���̬��Ϊ����̬����Ԫ�ؿ��ܱ�������Ҳ���ܱ���ԭ |
| D��ʧ����Խ�ԭ��Խǿ���õ���Խ��������Խǿ |
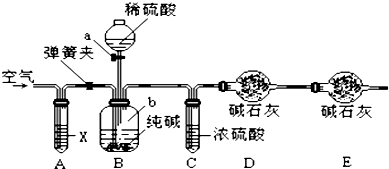
 ��1���������¡��˹��̵������о��������ڳ��³�ѹ����������N2�ڴ���������ˮ������Ӧ��2N2��g��+6H2O��l��=4NH3��g��+3O2��g��������֪��
��1���������¡��˹��̵������о��������ڳ��³�ѹ����������N2�ڴ���������ˮ������Ӧ��2N2��g��+6H2O��l��=4NH3��g��+3O2��g��������֪��