��Ŀ����
Ϊ�ⶨij�����Ͻ�����������������ij�о���ѧϰС�����������ʵ��������̣���ȡ�úϽ�1.0g�������ձ��м��������ᣬ���ϱ�����ʹ���ַ�Ӧ����Ӧֹͣ������ȴ�����м������NaOH��Һ����ַ��ú���ˣ����˳��ij�����ϴ���ڿ����м������������أ��õ�������1.2g���Իش�
��1��ʵ�����Ա�������ձ���ԭ�� ��
��2���������NaOH��Һ������ ��
��3������NaOH��Һ���־��õ����� ��
��4��ʵ����ϴ�ӳ�������Ҫ�IJ��������� ���ж��ϱ������г����Ƿ�ϴ�����õ��Լ��� ����������ʱ������ʢ�Ź�������������� ��
��5������������Ǽ���Ͻ��������������� ��
��6����һ�룬������ʲô�������ⶨAl��������������д��һ�ַ������ɣ� ��
��1��ʵ�����Ա�������ձ���ԭ��
��2���������NaOH��Һ������
��3������NaOH��Һ���־��õ�����
��4��ʵ����ϴ�ӳ�������Ҫ�IJ���������
��5������������Ǽ���Ͻ���������������
��6����һ�룬������ʲô�������ⶨAl��������������д��һ�ַ������ɣ�
���㣺̽�����ʵ���ɻ�������ʵĺ���
ר�⣺ʵ��̽�������ݴ�����
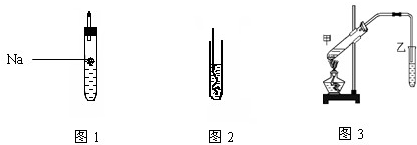
�����������Ͻ��м����������ᣬ�����Ȼ��������Ȼ�������������Ӧ����Һ�м��������������ƣ��������������γ����������������������γ�ƫ�����ƣ����ˣ��õ������������������յõ�������������������������������������������������������������
��1�������ڻ��ý����������ᷴӦ���ң���ֹҺ�彦����Ӱ��ⶨ�����
��2������ʵ��Ŀ�ķ�����������������Ƶ����ã�
��3���������ƺ������������������������������������������ȶ����ױ�����������������
��4������ϴ�Ӳ������������ݳ������渽�����ʣ���ȡ��Ӧ�ļ��鷽�������չ����������н��У�
��5�������������������������������������ɼ������������������������
��6�����ɸ��������������Ƹ��У���������Ӧ����Ʋⶨ���ķ�����
��1�������ڻ��ý����������ᷴӦ���ң���ֹҺ�彦����Ӱ��ⶨ�����
��2������ʵ��Ŀ�ķ�����������������Ƶ����ã�
��3���������ƺ������������������������������������������ȶ����ױ�����������������
��4������ϴ�Ӳ������������ݳ������渽�����ʣ���ȡ��Ӧ�ļ��鷽�������չ����������н��У�
��5�������������������������������������ɼ������������������������
��6�����ɸ��������������Ƹ��У���������Ӧ����Ʋⶨ���ķ�����
���
�⣺��1�������ڻ��ý����������ᷴӦ���ң���ֹҺ�彦����Ӱ��ⶨ��������ñ������ס�ձ����ʴ�Ϊ����ֹҺ�彦����Ӱ��ⶨ�����
��2����Ӧ����Һ�м��������������ƣ��������������γ����������������������γ�ƫ�����ƣ����ˣ��õ������������������յõ��������������������������������������������������������������ʴ�Ϊ��ʹ��Ԫ��ȫ��������Һ���������������룻
��3���������ƺ������������������������������������������ȶ����ױ���������������������NaOH��Һ���־��õ����ã��DZ�֤�����������������е������������Ϊ������������Ӧ����ʽΪ��4Fe��OH��2+O2=4Fe��OH��3���ʴ�Ϊ����֤�����������������е������������Ϊ����������
��4��ϴ�ӳ����ڹ������н��У��������Ϊ���ò���������ע������ˮ����û��������ˮ��Ȼ���£��ظ�����2-3�Σ������ò�������Ϊ���ձ�����������©�������������������渽�����������ӣ��ɼ������һ��ϴҺ���Ƿ���������ӣ����巽��Ϊ��ȡ���һ��ϴҺ���������������ữ�������������������ɣ�֤��ϴ�Ӹɾ������չ����������н��У��ʴ�Ϊ���ձ�����������©���������ữ����������Һ��������
��5��1.2g����Ϊ���������������������������ʵ���Ϊ��
=0.0075mol���������ʵ���Ϊ��0.015mol������Ϊ��0.015mol��56g/mol=0.84g����������Ϊ��1.0g-0.84g=0.16g��������������Ϊ��
��100%=16%���ʴ�Ϊ��16%��
��6�������������Ʒ�Ӧ���������������Ʋ���Ӧ���ʿ����������ƺ���Ʒ��Ӧ��ͨ��������Ӧ��ʣ������������������������������������巽��Ϊ����һ�������ĺϽ��ܽ��ڹ���������������Һ�У����ˡ�ϴ�ӡ�����ù��壬���Ƶ�������Ϊ�����������Ӷ�������������������õ���������������
�ʴ�Ϊ����һ�������ĺϽ��ܽ��ڹ���������������Һ�У����ˡ�ϴ�ӡ�����ù��壬���Ƶ�������Ϊ�����������Ӷ�������������������õ���������������
��2����Ӧ����Һ�м��������������ƣ��������������γ����������������������γ�ƫ�����ƣ����ˣ��õ������������������յõ��������������������������������������������������������������ʴ�Ϊ��ʹ��Ԫ��ȫ��������Һ���������������룻
��3���������ƺ������������������������������������������ȶ����ױ���������������������NaOH��Һ���־��õ����ã��DZ�֤�����������������е������������Ϊ������������Ӧ����ʽΪ��4Fe��OH��2+O2=4Fe��OH��3���ʴ�Ϊ����֤�����������������е������������Ϊ����������
��4��ϴ�ӳ����ڹ������н��У��������Ϊ���ò���������ע������ˮ����û��������ˮ��Ȼ���£��ظ�����2-3�Σ������ò�������Ϊ���ձ�����������©�������������������渽�����������ӣ��ɼ������һ��ϴҺ���Ƿ���������ӣ����巽��Ϊ��ȡ���һ��ϴҺ���������������ữ�������������������ɣ�֤��ϴ�Ӹɾ������չ����������н��У��ʴ�Ϊ���ձ�����������©���������ữ����������Һ��������
��5��1.2g����Ϊ���������������������������ʵ���Ϊ��
| 1.2g |
| 160g/mol |
| 0.16g |
| 1.0g |
��6�������������Ʒ�Ӧ���������������Ʋ���Ӧ���ʿ����������ƺ���Ʒ��Ӧ��ͨ��������Ӧ��ʣ������������������������������������巽��Ϊ����һ�������ĺϽ��ܽ��ڹ���������������Һ�У����ˡ�ϴ�ӡ�����ù��壬���Ƶ�������Ϊ�����������Ӷ�������������������õ���������������
�ʴ�Ϊ����һ�������ĺϽ��ܽ��ڹ���������������Һ�У����ˡ�ϴ�ӡ�����ù��壬���Ƶ�������Ϊ�����������Ӷ�������������������õ���������������
���������⿼���������Ͻ�����Ԫ�����������IJⶨ���е��Ѷȣ�ע������ⶨԭ���ͻ���������

��ϰ��ϵ�д�
�����Ŀ
����ɻ��������������泥�NH4ClO4���Ļ����Ϊ����ȼ�ϣ���ȼʱ���۱��������ų�����������������立�Ӧ���䷽��ʽ�ɱ�ʾΪ��2NH4ClO4��s���TN2��g��+4H2O��g��+Cl2��g��+2O2��g����H��0��
���жԸ÷�Ӧ������������ǣ�������
���жԸ÷�Ӧ������������ǣ�������
| A���÷�Ӧ���ڷֽⷴӦ |
| B����Ӧ˲������������������ƶ�����ɻ����� |
| C���÷�Ӧ��Ҫ�ǽ���ѧ��ת��Ϊ���ܺͶ��� |
| D���ڷ�Ӧ�и������ֻ�������� |