��Ŀ����
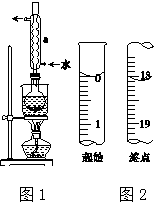
15��������һ���������Һ�У����в���ʹ�õ�����ҺŨ��ƫ�ߡ�ƫ�ͻ�����Ӱ�죿��1�����ܽ����ʵ��ձ��ڵ�Һ�嵹������ƿ��δϴ���ձ��ͽ��ж��ݣ�ƫ�ͣ�
��2������ʱ��Һ�泬������ƿ���ϵĿ̶��ߣ��ý�ͷ�ιܽ�������Һ��������ƫ�ͣ�
��3������ҡ�Ⱥ���ƿ��Һ���Ե���ƿ���̶��ߣ���Ӱ�죮
��4������ϡ����ʱ�������õ�Ũ���᳤ʱ��������ܷⲻ�õ������У���ʹ��ҺŨ��ƫ�ͣ�
���� �������������ʵ����ʵ���n����Һ�����V������C=$\frac{n}{V}$����������������ʹnƫ�����ʹVƫС�IJ�������ҺŨ��ƫ�ߣ�����ʹnƫС����Vƫ��IJ�������ҺŨ��ƫ�ͣ�
��� �⣺��1�����ܽ����ʵ��ձ��ڵ�Һ�嵹������ƿ��δϴ���ձ��ͽ��ж��ݣ��ᵼ��n��С����ҺŨ��ƫ�ͣ�
��2������ʱ��Һ�泬������ƿ���ϵĿ̶��ߣ��ý�ͷ�ιܽ�������Һ��������Ҳ���������ʣ��ᵼ��n��С����ҺŨ��ƫ�ͣ�
��3������ҡ�Ⱥ���ƿ��Һ���Ե���ƿ���̶��ߣ���ʵ������Ӱ�죻
��4������ϡ����ʱ�������õ�Ũ���᳤ʱ��������ܷⲻ�õ������У�Ũ������ˮ����ʹ��ҺŨ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�ƫ�ͣ���Ӱ�죻ƫ�ͣ�
���� ���⿼����һ�����ʵ���Ũ����Һ�������ķ�������ȷ����ԭ������C=$\frac{n}{V}$���ɽ����Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
5��һ���ǿ�����ȡ�����ᣬ���Ǹ��ֽⷴӦ�Ĺ���֮һ����֪�ڳ����²��Ũ�Ⱦ�Ϊ0.1mol•L-1������6����Һ��pH��
��1���������������Ϣ�ж����з�Ӧ���ܷ�������CE�����ţ���
A��CH3COOH+NaCN�TCH3COONa+HCN
B��CO2+H2O+C6H5ONa-��NaHCO3+C6H5OH
C��2HCN+Na2CO3�T2NaCN+CO2+H2O
D��Na2CO3+C6H5OH-��NaHCO3+C6H5ONa
E��CO2+H2O+2NaClO�TNa2CO3+2HClO
����֪HA��H2B���������ᣬ�������¹�ϵ��H2B��������+2A-�TB2-+2HA����A-��B2-��HB-���������ӽ��H+������˳��ΪA-��B2-��HB-��
��2��һЩ���ֽⷴӦ�ķ�������ѭ�������ɣ����б仯�����ڸ��ֽⷴӦ��
�ٽ�ʯ�����봿����Һ��Ͽ��Ƶÿ�������Һ��
����̼�������Һ�м��뱥��ʳ��ˮ�ɻ��С�մ���塡
������KCl��NaNO3�Ļ����Һ����������NaCl���壮
����������Ӧ���ܽ�����ֽⷴӦ��������һ����Ϊ���ܽ����Խϴ�������������ܽ����Խ�С�����ʵķ�����У�
��3��������ij������ܽ���ˮ�к���Һ�е�c��H+��=10-9 mol•L-1����õ���ʿ�����AD������ţ�
A��Na2S B��HCl C��K2SO4 D��NaOH E��CuSO4
��4�������£���pH=3������a L�ֱ�������������Һ��ϣ������Һ�������ԣ�
��Ũ��Ϊ1.0��10-3 mol•L-1�İ�ˮb L��
��c��OH-��=1.0��10-3 mol•L-1�İ�ˮc L��
��c��OH-��=1.0��10-3 mol•L-1������������Һd L��
��a��b��c��d֮���ɴ�С��˳���ǣ�b��a=d��c��
��5��һ���¶��£������������ʵ���Ũ�ȵ�����������Һ����NaOH����CH3COOH����CH3COONa�ֱ�ӵ���ˮ��pH�仯��С���Ǣۣ����ţ���һ���¶��£���������ˮ�зֱ��������ʵ�����CH3COONa��NaCN������Һ�������ӵ������ʵ����ֱ�Ϊn1��n2����n1��n2�Ĺ�ϵΪn1��n2�����������������=������
| ���� | CH3COONa | NaHCO3 | Na2CO3 | NaClO | NaCN | C6H5ONa |
| pH | 8.1 | 8.8 | 11.6 | 10.3 | 11.1 | 11.3 |
A��CH3COOH+NaCN�TCH3COONa+HCN
B��CO2+H2O+C6H5ONa-��NaHCO3+C6H5OH
C��2HCN+Na2CO3�T2NaCN+CO2+H2O
D��Na2CO3+C6H5OH-��NaHCO3+C6H5ONa
E��CO2+H2O+2NaClO�TNa2CO3+2HClO
����֪HA��H2B���������ᣬ�������¹�ϵ��H2B��������+2A-�TB2-+2HA����A-��B2-��HB-���������ӽ��H+������˳��ΪA-��B2-��HB-��
��2��һЩ���ֽⷴӦ�ķ�������ѭ�������ɣ����б仯�����ڸ��ֽⷴӦ��
�ٽ�ʯ�����봿����Һ��Ͽ��Ƶÿ�������Һ��
����̼�������Һ�м��뱥��ʳ��ˮ�ɻ��С�մ���塡
������KCl��NaNO3�Ļ����Һ����������NaCl���壮
����������Ӧ���ܽ�����ֽⷴӦ��������һ����Ϊ���ܽ����Խϴ�������������ܽ����Խ�С�����ʵķ�����У�
��3��������ij������ܽ���ˮ�к���Һ�е�c��H+��=10-9 mol•L-1����õ���ʿ�����AD������ţ�
A��Na2S B��HCl C��K2SO4 D��NaOH E��CuSO4
��4�������£���pH=3������a L�ֱ�������������Һ��ϣ������Һ�������ԣ�
��Ũ��Ϊ1.0��10-3 mol•L-1�İ�ˮb L��
��c��OH-��=1.0��10-3 mol•L-1�İ�ˮc L��
��c��OH-��=1.0��10-3 mol•L-1������������Һd L��
��a��b��c��d֮���ɴ�С��˳���ǣ�b��a=d��c��
��5��һ���¶��£������������ʵ���Ũ�ȵ�����������Һ����NaOH����CH3COOH����CH3COONa�ֱ�ӵ���ˮ��pH�仯��С���Ǣۣ����ţ���һ���¶��£���������ˮ�зֱ��������ʵ�����CH3COONa��NaCN������Һ�������ӵ������ʵ����ֱ�Ϊn1��n2����n1��n2�Ĺ�ϵΪn1��n2�����������������=������
6�������������Ȼ�ѧ����ʽ����ȷ���ǣ�������
| A�� | ��֪C2H6��ȼ����Ϊ1090 kJ•mol-1����C2H6ȼ�յ��Ȼ�ѧ����ʽΪ��C2H6��g��+3.5O2��g���T2CO2��g��+3H2O��g����H=-1090 kJ/mol | |
| B�� | 25�棬101kPa�£�l mol C6H6ȼ������CO2��Һ̬ˮʱ�ų�3260 kJ���������Ȼ�ѧ����ʽΪ��C6H6��g��+7.5O2��g��=6CO2��g��+3H2O ��l����H=-3260kJ•mol-1 | |
| C�� | ��֪�����£�H+��aq��+OH-��aq���TH2O��l����H=-57.3 kJ/mol����ϡ������ϡNaOH��Һ��Ӧ����1 mol H2O��l��ʱҲ�ų�57.3 kJ������ | |
| D�� | ��֪2CO��g��+O2��g���T2CO2��g����H=-566 kJ•mol-1����CO��ȼ���ȡ�H=-283 kJ•mol-1 |
10����һ��������MnO4-��SO32-��Ӧ������Mn2+������1molMnO4-��Ӧ����SO32-�����ʵ���Ϊ��������
| A�� | 3.0mol | B�� | 2.5mol | C�� | 1.0mol | D�� | 0.75mol |
7��ԭ������������������ֶ�����Ԫ��X��Y��Z��W��XԪ����ɵĵ�������Ȼ����Ӳ�����YԪ�ص�ԭ�������������Ǵ�����������������WԪ����YԪ����ɵĻ�����������ᷴӦ������Ӧ��ZԪ�ص�ԭ�������ֻ��һ�����ӣ���������������ǣ�������
| A�� | Ԫ�ؽ����ԣ�Z��W | |
| B�� | X��Y���γɶ��ֹ��ۻ����� | |
| C�� | ������Z2Y2�мȺ����Ӽ����ֺ����ۼ� | |
| D�� | YԪ��ֻ���γ�һ�ֵ��� |
5����֪HF�����Ա�HCN������ǿ���������ʵ���Ũ�Ⱥ��������ͬ��NaF��NaCN������Һ����֪ǰ����Һ�е�������ĿΪn1��������Һ�е�������ĿΪn2�������й�ϵ��ȷ���ǣ�������
| A�� | n1=n2 | B�� | n1��n2 | C�� | n1��n2 | D�� | C�� F-����C��CN-�� |
 Na2S2O3����Ҫ�Ļ���ԭ�ϣ�������ˮ�������Ի���Ի������ȶ���
Na2S2O3����Ҫ�Ļ���ԭ�ϣ�������ˮ�������Ի���Ի������ȶ��� +NaOH$\stackrel{��}{��}$
+NaOH$\stackrel{��}{��}$ +Cu2O+3H2O��ʵ�����ô˷�����ȡ���������Cu2O���壬��Ҫ�IJ����������Թܡ��ƾ��ơ��ձ�����ͷ�ι��⣬����Ҫ©����������
+Cu2O+3H2O��ʵ�����ô˷�����ȡ���������Cu2O���壬��Ҫ�IJ����������Թܡ��ƾ��ơ��ձ�����ͷ�ι��⣬����Ҫ©����������