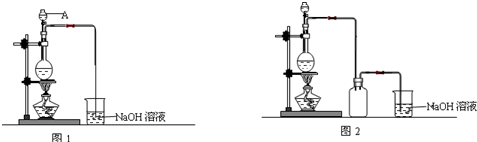
��Ŀ����
4��������ͭ�Ǵ���ˮ�潢ͧ����Ϳ�����Ҫԭ�ϣ�ijС��ͨ���������ϣ�������ͼ�о���I��Cu2O����ȡ
���ϣ�
1��������ͭ����ѧʽCu2O����ɫ�����ɫ�ᾧ���ĩ��������ˮ���л��ܼ���������ϡ���ᡢϡ����ȣ���ϡ�������绯Ϊ����ͭ��ͭ���ʣ�������ͭ��1800��ʱ�ֽ⣬�ڸ���������ȶ������ڳ�ʪ�����б���������Ϊ2������ͭ��������ͭ��Ҫ�������촬�����ᡢɱ�����
�����ǻ�ԭ���Ʊ�������ͭ����������������������ͭ����Һ��Ϻ���з�Ӧ������������ͭ���������Ʋ���ʱ������������ͭ���ɣ�
��1�������ǻ�ԭ����Cu2O�Ļ�ѧ����ʽΪ
 +NaOH$\stackrel{��}{��}$
+NaOH$\stackrel{��}{��}$ +Cu2O+3H2O��ʵ�����ô˷�����ȡ���������Cu2O���壬��Ҫ�IJ����������Թܡ��ƾ��ơ��ձ�����ͷ�ι��⣬����Ҫ©����������
+Cu2O+3H2O��ʵ�����ô˷�����ȡ���������Cu2O���壬��Ҫ�IJ����������Թܡ��ƾ��ơ��ձ�����ͷ�ι��⣬����Ҫ©����������������Ʒ���Ƿ���CuO
����1�����Ƶõ�Cu2O��Ʒ��������ϡ���ᣮ
��2����ͬѧ��Ϊ����Һ��������˵����Ʒ�к���CuO���ʣ���ͬѧ��Ϊ�������������������ӷ�Ӧ����ʽ����ԭ��Cu2O+H2SO4=CuSO4+Cu+H2O��
��3����ͬѧͨ����˼����Ϊ�����Լ����Ϊ�����ⶨ����ȷ����Ʒ���Ƿ���CuO���ʣ��ڷ�����1��ʵ������ϼ��ٶ����о���Ӧ������������Cu2O��Ʒ����������Ӧ��ʣ����壨Cu��������
����2����ͬѧ��Ϊ��������װ�ã�����ҩƷ������������ʵ�飬ͨ���ⶨcװ�÷�Ӧ�����������Լ�dװ�÷�Ӧǰ�����ص��������м��㣬�Ӷ�ȷ����Ʒ���Ƿ�������ͭ��

��4��װ��a�����ӵ�����H2SO4���ѧʽ����װ��e�м�ʯ�ҵ������Ƿ�ֹ�����е�ˮ����dװ�ã�m��H2O���ⶨ��ȷ��
��5����ȼװ��c�оƾ���֮ǰ����еIJ����Ǵ�K1���ر�K2��ͨһ��ʱ���������鴿���ٴ�K2���ر�K1��
��6��Ϩ��ƾ���֮������ͨһ��ʱ��������Ӳ�ʲ�������ȴ��ԭ���Ƿ�ֹ���ɵ�Cu�����ڸ������ֱ���������ΪCuO�����²ⶨ���ݲ�ȷ��
���� ��1��������������������ͭ��Ӧ��Ҫ���ȣ�����������ͭ��������Ҫ�������˲������з��룻
����1����2��������ͭҲ�����ᷢ��������ԭ��Ӧ��������ͭ����Һ����ɫ��ͬʱ����Cu���ʣ�
��3�������Լ����Ϊ�����ⶨ����ȷ����Ʒ���Ƿ���CuO���ʣ�Ӧ������������������ͭ��Ʒ�����ͷ�Ӧ����ͭ��������ͨ����ѧ����ʽ���������
����2��aװ�ò���������bװ�ø���������cװ����������ͭ�������ﷴӦ�û���Cu��dװ������ˮ�����Բⶨ����ˮ��������װ��e�м�ʯ�ҵ������Ƿ�ֹ�����е�ˮ����dװ�ã�Ӱ��ˮ�����ⶨ��
��4��aװ�ò���������Ӧ��ֹ��Ļӷ���Ӧѡ���ѻӷ��Ե��
��5����ȼװ��c�оƾ���֮ǰ����������Ĵ��ȣ�
��6��Ϩ��ƾ���֮������ͨһ��ʱ��H2���Թ���ȴ�Ƿ�ֹ���������������ɵ�ͭ���ⶨ���������
��� �⣺��1��������������������ͭ��Ӧ��Ҫ���ȣ������貣������Ϊ�Թܡ��ƾ��ƣ��������ɵ�������ͭ��������Ҫ���й��˲��������貣������Ϊ©�������������ձ���
�ʴ�Ϊ��©������������
����1����2��������ͭҲ�����ᷢ��������ԭ��Ӧ��������ͭ����Һ����ɫ��ͬʱ����Cu���ʣ���Ӧ�Ļ�ѧ����ʽΪ��Cu2O+H2SO4=CuSO4+Cu+H2O��
�ʴ�Ϊ��Cu2O+H2SO4=CuSO4+Cu+H2O��
��3�������Լ����Ϊ�����ⶨ����ȷ����Ʒ���Ƿ���CuO���ʣ�Ӧ������������Cu2O��Ʒ����������Ӧ��ʣ����壨Cu����������
�ʴ�Ϊ��Cu2O��Ʒ����������Ӧ��ʣ����壨Cu����������
����2��aװ�ò���������bװ�ø���������cװ����������ͭ�������ﷴӦ�û���Cu��dװ������ˮ�����Բⶨ����ˮ��������װ��e�м�ʯ�ҵ������Ƿ�ֹ�����е�ˮ����dװ�ã�Ӱ��ˮ�����ⶨ��
��4��dװ���еļ�ʯ�һ�����ˮ���������壬��ֹa����ӷ�����ѡ�ѻӷ��������ᣬװ��e�м�ʯ�ҵ������Ƿ�ֹ�����е�ˮ����dװ�ã�m��H2O���ⶨ��ȷ��
�ʴ�Ϊ��H2SO4����ֹ�����е�ˮ����dװ�ã�m��H2O���ⶨ��ȷ��
��5����ȼװ��c�оƾ���֮ǰ����������Ĵ��ȣ��鴿��Ӧ����������ͨ��dװ�ã�ʹ���ɵ�ˮȫ����dװ�����գ������������K1���ر�K2��ͨһ��ʱ���������鴿���ٴ�K2���ر�K1��
�ʴ�Ϊ����K1���ر�K2��ͨһ��ʱ���������鴿���ٴ�K2���ر�K1��
��6�����ȵ�ͭ�������ᷢ����Ӧ��������ͭ������Ϩ��ƾ���֮������ͨһ��ʱ��H2���Թ���ȴ�Ƿ�ֹ���������������ɵ�ͭ���ⶨ���������
�ʴ�Ϊ����ֹ���ɵ�Cu�����ڸ������ֱ���������ΪCuO�����²ⶨ���ݲ�ȷ��
���� ���⿼��ʵ���Ʊ��������Ƕ�ѧ���ۺ������Ŀ��飬���ؿ���ѧ����ԭ����װ�÷������ۣ��Ѷ��еȣ�

 ���б�ˢ��ϵ�д�
���б�ˢ��ϵ�д�| A�� | 0.01mol/L NH4Al��SO4��2��Һ��0.01mol•L-1Ba��OH��2��Һ��������NH4++Al3++2SO42-+2Ba2++4OH-=2BaSO4��+Al��OH��3��+NH3•H2O | |
| B�� | �ö��Ե缫���CuCl2��Һ��2Cu2++2H2O$\frac{\underline{\;���\;}}{\;}$2Cu+O2��+4H+ | |
| C�� | ����״���µ�11.2L����ͨ��200mL2mol•L-1��FeBr2��Һ�У����ӷ�Ӧ����ʽΪ��4Fe2++6Br-+5Cl2=4Fe3++3Br2+10Cl- | |
| D�� | �����еμ�����Ũ���Fe+3NO3-+6H+=Fe3++3NO2��+3H2O |
| A�� | ����Һ�У�c2��H+����c��H+��•c��A-��+Kw | |
| B�� | 0.1mol•L-1 HA��Һ��0.05mol•L-1 NaOH��Һ�������ϣ�������Һ�У�2c��H+��+c��HA���Tc��A-��+2c��OH-�� | |
| C�� | Ũ�Ⱦ�Ϊ0.1mol•L-1��HA��NaA��Һ�������ϣ�������Һ�У�c��A-����c��HA����c��Na+����c��OH-����c��H+�� | |
| D�� | ��pH=3��HA��Һ��pH=11��NaOH��Һ�������ϣ�������Һ�У�c��Na+����c��A-����c��OH-����c��H+�� |
 ��Ȼά����P���ṹ��ͼ�����ӽṹ��RΪ���������������ڻ��������У�����һ��Ӫ��������������ά����P��������ȷ���ǣ�������
��Ȼά����P���ṹ��ͼ�����ӽṹ��RΪ���������������ڻ��������У�����һ��Ӫ��������������ά����P��������ȷ���ǣ�������| A�� | 1mol�����ʿ���5molNaOH������Ӧ | |
| B�� | 1mol��������������ˮ��Ӧ���������6molBr2 | |
| C�� | һ��������1mol�����ʿ���H2�ӳɣ�����H2�����Ϊ6mol | |
| D�� | ά����P�ܷ���ˮ�ⷴӦ |