��Ŀ����
20������98%��Ũ���ᣨ��=1.84g•cm-3�����Ƴ�Ũ��Ϊ0.5mol•L-1��ϡ����450mL����1��ѡ�õ���Ҫ�����У���Ͳ���ձ�����������500mL����ƿ����ͷ�ιܣ�
��2���뽫���и�����������ȷ��������ں����ϣ�
A������Ͳ��ȡŨH2SO4
B�������ߵ�ҡ��
C���ý�ͷ�ιܼ�����ˮ���̶���
D��ϡ��ŨH2SO4
E������Һת������ƿ
�������ȷ��˳������ΪA��D��E��C��B��
��3����Ҫ�ش��������⣺
��98%��Ũ�������ʵ���Ũ��Ϊ18.4 mol/L����ҪŨ��������Ϊ13.6mL��
�����ʵ������15mL��20mL��50mL����ͲӦѡ��15mL����Ͳ��ã���ȡʱ������Ͳ���ɾ���ˮϴ����ֱ����ȡ��ʹŨ��ƫ�ͣ��ƫ�ߡ�����ƫ�͡�������Ӱ�족����ͬ��
�۽�Ũ�������ձ��ڱ�����ע��ʢˮ���ձ��У����ò��������Ͻ����Ŀ���Ƿ�ֹ���У�Ѹ��ɢ�ȣ��������������Һ�彦�������ʹŨ��ƫ�ͣ�
����ת������ƿǰ�ձ���Һ��Ӧ����ȴ�������ʹŨ��ƫ�ߣ�����ʱ����
ʹ��Һ��Һ����̶������У������ӻ�ʹŨ��ƫ�ߣ�
���� ��1���������Ʋ����Ǽ��㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��������Ҫ��������
��2����������һ�����ʵ���Ũ����Һ��һ�㲽����
��3���ٸ���c=$\frac{1000�Ѧ�}{M}$�����98%��Ũ���ᣨ��=1.84g•cm-3�������ʵ���Ũ�ȣ�����ϡ��ǰ����Һ�����ʵ����ʵ������������ҪŨ����������
�ڸ�����ȡŨ����������ѡ������Ͳ�Ĺ����ӽ�����Ͳ��������ȡ��Ũ�����൱�ڱ�ϡ���˷�����
��Ũ����ϡ�����зų��������У������������Һ�彦������ʹ���Ƶ���Һ�����ʼ�С��
����ת������ƿǰ�ձ���Һ��δ��ȴ�����ƫ����ȴ�����ƫС������ʱ���ӣ���Һ��Һ���ڿ̶������£�������Һ���ƫС�����c=$\frac{n}{V}$�жϣ�
��� �⣺�ٲ��������м��㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ������Ͳ��ȡ�����ձ����ܽ⣬���ò��������裬�����ܽ⣮��ȴ��ת�Ƶ�500mL����ƿ�У���450ml��������ƿ�������ò�����������ϴ���ձ���������2-3�Σ�����ϴ��Һ��������ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ���������IJ�����������Ͳ���ձ�����������500mL����ƿ����ͷ�ιܣ������ṩ��������֪������������500mL����ƿ��
�ʴ�Ϊ��500mL����ƿ��
��2������500mL 0.5mol/L��ϡ������Һ�IJ���Ϊ�����㡢�������ܽ⡢ת�ơ�ϴ�ӡ����ݡ�ҡ�ȵȣ�������ȷ������˳��ΪA��D��E��C��B��
�ʴ�Ϊ��A��D��E��C��B��
��3����98%��Ũ���ᣨ��=1.84g•cm-3�������ʵ���Ũ��Ϊ��c=$\frac{1000�Ѧ�}{M}$=$\frac{1000��1.84��98%}{98}$mol/L=18.4mol/L��ϡ��ǰ����Һ�����ʵ����ʵ������䣬����ҪŨ�������ΪV����V��18.4mol/L=0.5mol/L��500mL����ã�V=13.6ml��
�ʴ�Ϊ��18.4 mol/L��13.6��
����ȡ13.6mLŨ���ᣬ��Ҫʹ��15mL����Ͳ����ȡʱ������Ͳ���ɾ���ˮϴ����ֱ����ȡ��Ũ���ᱻ����ˮϡ�ͣ�����Ũ�����Ũ��ƫ�ͣ�
�ʴ�Ϊ��15��ƫ�ͣ�
��Ũ����ϡ�ͷ��ȣ���Һ���¶����ߣ�ת��ǰ������ȴϡ�͵���Һ�����������Ƶ���Һ�¶Ƚϸ߱��У����ƫ����ȴ�����Ƶ���Һ�����ƫ�ͣ����յ���Ũ��ƫ�ߣ��������������Һ�彦�����������ʵ�����С��Ũ��ƫ�ͣ�
�ʴ�Ϊ����ֹ���У�Ѹ��ɢ�ȣ�ƫ�ͣ���
����ת������ƿǰ�ձ���Һ��Ӧ����ȴ��δ��ȴ���ƫ���ݺ���ȴ���ƫС��Ũ��ƫ����ʱ���ӿ̶��ߣ�����������Һ��Һ�����ƫС��Ũ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�ƫ�ߣ�
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���Ŀ�ѶȲ�����c=$\frac{n}{V}$��������Һ����ԭ������������ע��Ũ�����ϡ�ͷ�����

 ���ݼ���ϵ�д�
���ݼ���ϵ�д�
| A�� | ͼ����ʾ��ʵ�飺������Һ��ɫ�仯�ɱȽ�Zn��Cu�Ľ������ | |
| B�� | ͼ����ʾ��ʵ�飺����С�Թ���Һ��ı仯�ж������������ⸯʴ | |
| C�� | ͼ����ʾ��ʵ�飺�����¶ȼƶ����ı仯��Ũ�����Na0H��Ӧ�ⶨ�к��� | |
| D�� | ͼ����ʾ��ʵ�飺��������ƿ��������ɫ�ı仯�ж�2N02��g��?N20��g�������ȷ�Ӧ |
| ��� | Cu | Zn | S |
| 1 | 10.3% | 5.0% | 1.2% |
| 2 | 11.5% | 4.9% | 1.8% |
| 3 | 12.4% | 10.3% | 0.9% |
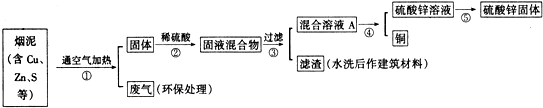
��1��д������٢��к�ͭԪ�ص����ʷ�����Ӧ�Ļ�ѧ����ʽ��2Cu+O2$\frac{\underline{\;����\;}}{\;}$2CuO��CuO+H2SO4=CuSO4+H2O��
��2��д���������д��������ķ������û�ѧ����ʽ��ʾ����2NaOH+SO2=Na2SO3+H2O��
��3������������õIJ��������ǣ������ᾧ����������Ũ�����ᾧ����
��4���ڲ�����У���ѡ���Լ�Zn�ӻ����ҺA�еõ�ͭ��
| A�� | 0.1mol/LCH3COOH��Һ����ˮϡ�����У���������Ũ�Ⱦ���С | |
| B�� | Ũ�Ⱦ�Ϊ0.1mol/L��NaF��CH3COONa��Һ��Ƚϣ�CH3COONa��ҺpH�� | |
| C�� | ��ӦHF+CH3COONa�TNaF+CH3COOH���Է��� | |
| D�� | NaF��Һ�м�����NaOH���壬��Һ��c��F-����� |
 25��ʱ���������ʵĵ���ƽ�ⳣ�������ʾ��
25��ʱ���������ʵĵ���ƽ�ⳣ�������ʾ��| ��ѧʽ | CH3COOH | H2CO3 | HClO |
| ����ƽ�ⳣ�� | 1.7��10-5 | K1=4.3��10-7 K2=5.6��10-11 | 3.0��10-8 |
��1��CH3COOH��H2CO3��HClO��������ǿ������˳��ΪCH3COOH��H2CO3��HClO��
��2��ͬŨ�ȵ�CH3COO-��HCO${\;}_{3}^{-}$��CO${\;}_{3}^{2-}$��ClO-���H+��������ǿ������˳��ΪCO32-��ClO-��HCO3-��CH3COO-��
��3�����ʵ���Ũ�Ⱦ�Ϊ0.1mol•L-1�������������ʵ���Һ��a��Na2CO3��b��NaClO��c��CH3COONa��d��NaHCO3��pH�ɴ�С��˳����a��b��d��c�����ţ���
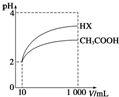
��4�����Ϊ10mL pH=2�Ĵ�����Һ��һԪ��HX�ֱ��ˮϡ����1000mL��ϡ������pH�仯��ͼ��ʾ����HX�ĵ���ƽ�ⳣ�����ڣ�����ڡ��������ڡ���С�ڡ�������ĵ���ƽ�ⳣ����������ϡ����ͬ������HX��pH�仯��CH3COOH�Ĵ�����ǿ������ƽ�ⳣ����ϡ�ͺ�HX��Һ����ˮ���������c��H+�����ڣ�����ڡ��������ڡ���С�ڡ���������Һ����ˮ���������c��H+
����������HX����ǿ��CH3COOH�ģ�ϡ�ͺ�HX��Һ�е�c��H+��С��CH3COOH��Һ�е�c��H+�����������ˮ�������������Ҳ������
| Ԫ�ش��� | A | B | C | D | E |
| ԭ�Ӱ뾶/nm | 0.186 | 0.143 | 0.089 | 0.104 | 0.074 |
| ��Ҫ���ϼ� | +1 | +3 | +2 | +6��-2 | -2 |
| A�� | BԪ�ز����γɹ��ۻ����� | |
| B�� | ���Ӱ뾶��СA+��D2- | |
| C�� | A��E�γɵĻ�������ֻ���ܺ����Ӽ� | |
| D�� | B��E�Ļ����ﲻ�����ڰ�ˮ |
